中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 赵弋戈, 郑平
- Yige Zhao, Ping Zheng
- 厌氧氨氧化体的组成、结构与功能
- Composition, structure and function of anammoxosome-A review
- 微生物学报, 2016,56(1): 8-18
- Acta Microbiologica Sinica, 2016,56(1): 8-18
-
文章历史
- 收稿日期:2015-03-26
- 修回日期:2015-03-26
厌氧氨氧化(Anammox)是在厌氧条件下,以亚硝酸为电子受体,以氨为电子供体产生氮气的生物反应[1, 2, 3]。该反应在自然界氮素循环中发挥着重要作用。据估计,Anammox所致的氮素转化量占海洋氮素转化总量的30%-50%[4]。该反应能同时脱除废水中的氨氮和亚硝氮,Anammox一经问世即受到环境工程界青睐。
厌氧氨氧化菌(AnAOB)是Anammox的功能载体,它是系统进化上分枝很深的浮霉状菌。通过持续20余年的研究,人们在浮霉菌门(Planctom-ycetes)中为AnAOB增设了新目——Candidatus Brocadiales[5]。不同于好氧氨氧化细菌(AOB),AnAOB细胞内拥有独特的细胞器——厌氧氨氧化体,它是AnAOB进行Anammox代谢的场所。本文综合国内外文献报道,对厌氧氨氧化体的组成、结构与功能作一综述,以期为从事Anammox研究的同行提供参考。
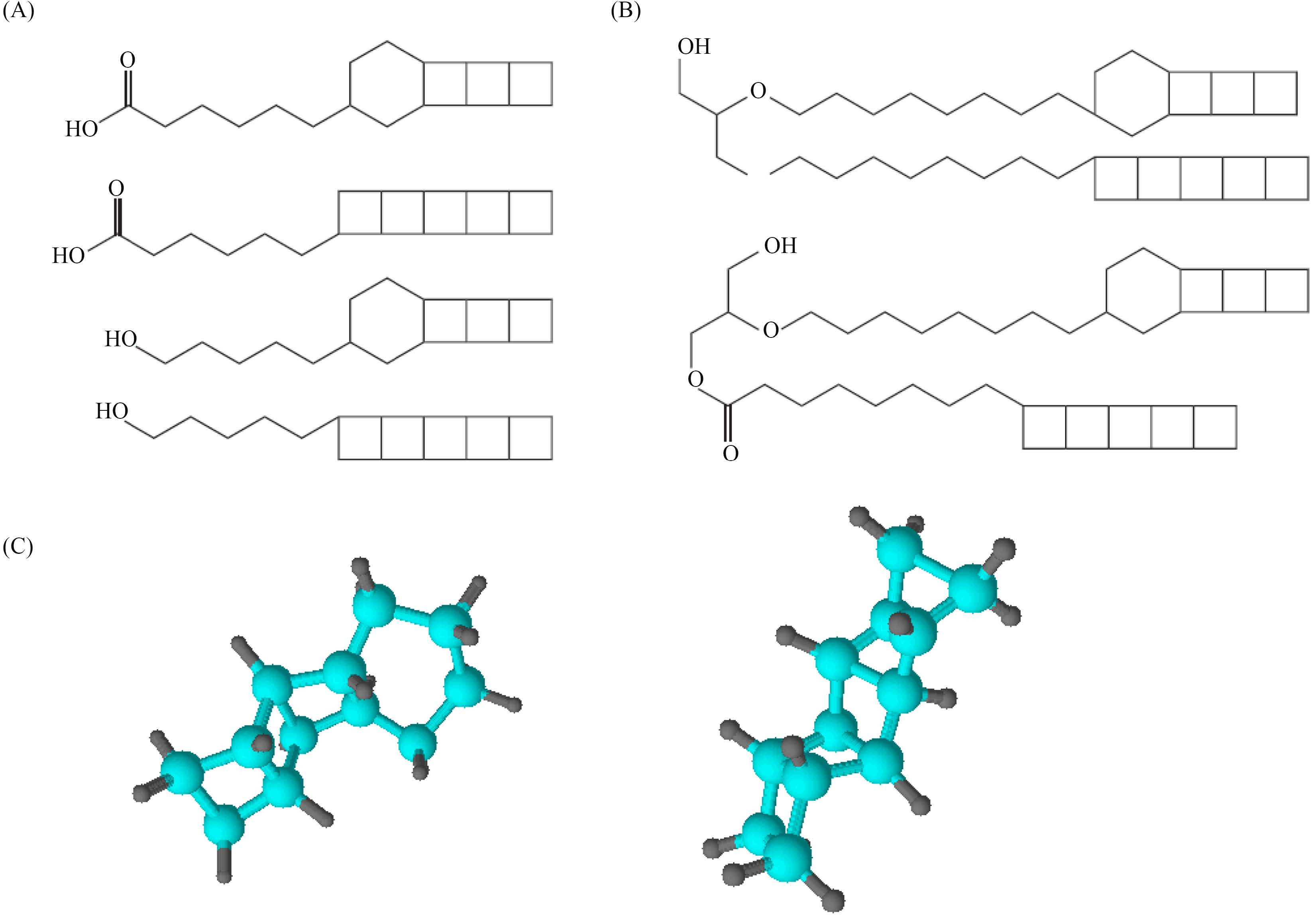
1 厌氧氨氧化体的组成和构造 1.1 厌氧氨氧化体膜的化学组成Damsté等[6]利用富集的Candidatus Brocadia anammoxidans研究厌氧氨氧化体膜,在富集过程中发现,厌氧氨氧化体中含有由多个稠合四元环烷烃组成的脂类。由于其线性串联的四元环环均为顺式排列,形状类似于阶梯(图1-C),因此称为梯形烷(ladderane)。梯形烷是AnAOB的特有成分,未见于其他细菌。脂质分析表明,梯形烷脂类占厌氧氨氧化体膜总脂质的53%,占细胞总脂质的34%。
研究表明,厌氧氨氧化体膜的C17-C20 梯形烷脂质含有3个线性串联的环丁烷和1个环己烷,或者5个串联的环丁烷(图1-A)。梯形烷脂肪酸通过酯键与甲醇或丙三醇连接,梯形烷醇则通过醚键与丙三醇相连,形成不同的脂质组合(图1-B)。其中,一种膜脂质确定为C20的脂肪酸甲酯,该脂肪酸甲酯结构包含1个庚基链,一端连接5个线性串联的环丁烷,另一端连接一个甲基酯。而另一种脂质确定为C20的sn-2-甘油单醚(sn-2-glycerol monoether),该sn-2-甘油单醚结构包含3个线性串联的环丁烷和1个环己烷并连接一个辛基链,在辛基链末端的碳原子上以醚键连接一个甘油。此外,还有一种更特殊的膜脂质确定为混合型甘油醚酯(图1-B),它包含以上两种脂质结构,混合型甘油醚酯未见于细菌和古菌。
|
| 图1 厌氧氨氧化体膜的梯形烷膜脂结构 Figure1 Ladderane and common membrane lipids of anammoxosome. (A) Ladderane fatty acid and ladderane fatty alcohol of unique membrane lipids of anammox bacteria; (B) General chemical structures of unique membrane lipids of anammox bacteria; (C) Two ball-and-stick models of ring structures of ladderane. Carbon and hydrogen atoms are represented by blue and grey balls, respectively. |
在不同的AnAOB中,梯形烷膜脂的种类和含量基本相似[7, 8, 9, 10],按其相对含量,梯形烷膜脂由多至少的排序为:脂肪酸甲酯、sn-2-烷基甘油单醚、醇、sn-1,2-二烷基甘油二醚、甘油醚和酰基甘油[11]。
在不同的AnAOB中,非梯形烷膜脂的种类和含量变化较大[12]。它们与梯形烷膜脂结合,以确保厌氧氨氧化体膜的抗泄漏性好于其他膜结构[6]。厌氧氨氧化体膜中的非梯形烷膜脂由直链脂肪酸、支链脂肪酸、单饱和脂肪酸和三萜系化合物组成。三萜系化合物包括C27的藿烷类化合物(hopanoid),细菌藿四醇(bacteriohopanetetrol,BHT)和鲨烯(squalene,C30H50)[13]。梯形烷膜脂曾被认为是厌氧氨氧化体膜所独有的成分。后来发现,梯形烷膜脂也存在于AnAOB的其他膜结构中,并能与非梯形烷膜脂结合而降低膜的渗透性[12]。
除了梯形烷,厌氧氨氧化体膜的脂类连接方式也值得关注。在古菌细胞膜中,主要成分为C20甘油醚和C40双甘油四醚,烃类通过醚键与甘油连接[14];在细菌细胞膜中,主要成分为磷脂、蛋白质和糖类,脂类通过醚键与甘油连接;而在厌氧氨氧化体膜中,脂类同时存在醚键和酯键两种连接方式(表1)。
| Type | Bacteria | Archaea | AnAOB |
| Chemical composition | Protein, lipids, saccharides | Protein, lipids, saccharides | Protein, lipids, saccharides |
| Main lipids | Polyglyceryl fatty,esters | Glycerol diether | Ladderane lipids |
| Lipid connection mode | Ester bond | Ether bond | Ester bond, ether bond |
由于梯形烷脂质的串联环烷烃顺式结构不能旋转,其三维结构排列成梯形(图1-C),致使厌氧氨氧化体膜的密度高于细菌和古菌的细胞膜[5],有助于防止Anammox代谢中间产物的逸散。到目前为止,梯形烷膜脂只见于AnAOB中,甚至未见于浮霉菌门的其他细菌[6]。因此,可将梯形烷脂质视作AnAOB的标志。
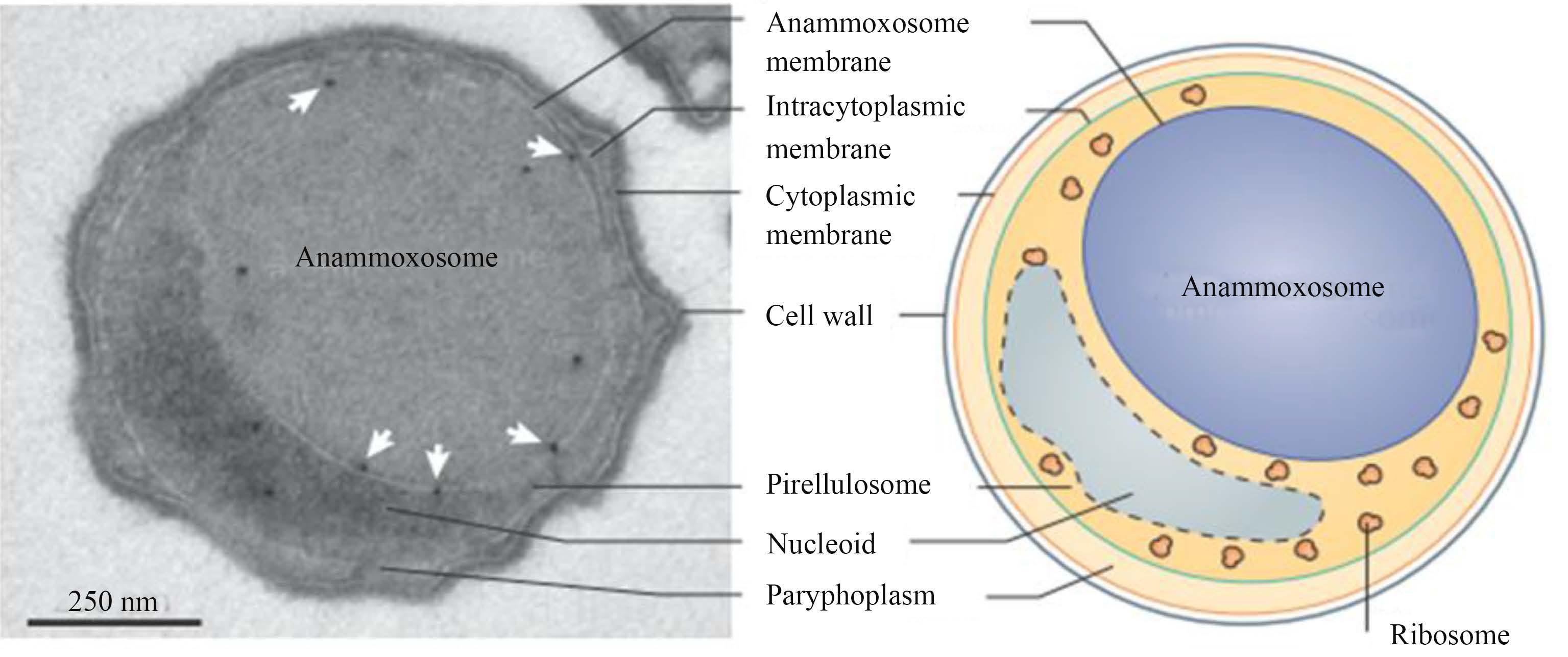
1.2 厌氧氨氧化体的基本构造厌氧氨氧化体由厌氧氨氧化体膜包围而成,位于AnAOB细胞中央,其体积占整个细胞的一半以上,其中不含核糖体。AnAOB的核糖体位于其核糖细胞质中 (图2)。厌氧氨氧化体膜内陷,形成褶皱或管状结构,深入厌氧氨氧化体内部[5]。
Van Niftrik等[16]利用电子断层扫描对厌氧氨氧化体进行三维成像显示,厌氧氨氧化体的双层膜多数呈弯曲状态,也有呈管状突起深入厌氧氨氧化体内部。透射电镜观察结果也显示,厌氧氨氧化体膜呈弯曲状态,但弯曲并不规则,有的膜部分弯曲,部分平直。虽然在不分裂的细胞中能观察到平直的膜,但在分裂的细胞中更易见到平直的膜。在细胞分裂期间,厌氧氨氧化体常常一侧被拉伸至无弯曲,而另一侧则保持弯曲状。Van Niftrik等[17]在用透射电镜观察Candidatus Kuenenia stuttgartiensis细胞时,发现厌氧氨氧化体呈椭球形,无弯曲。而在观察分裂的Candidatus B. fulgida细胞时,发现厌氧氨氧化体一端呈椭圆形无弯曲,而另一端则仍然保留弯曲状态。
Lindsay等[18]通过Candidatus K. stuttgartiensis超薄切片的电子显微镜照片发现,厌氧氨氧化体呈现中等电子密度(图3-A),并且具有很多颗粒结构,但没有核糖体状颗粒。纤维状拟核围绕着厌氧氨氧化体,并且有部分附着在厌氧氨氧化体膜上(图3-B)。通过anti-DNA抗体标记和二次抗体共轭15 nm胶体金的标记显示(图3-C),大多数DNA与纤维状拟核相关,存在于核糖细胞质中,但也有一些DNA出现在厌氧氨氧化体中。所有RNA都存在于核糖细胞质中。
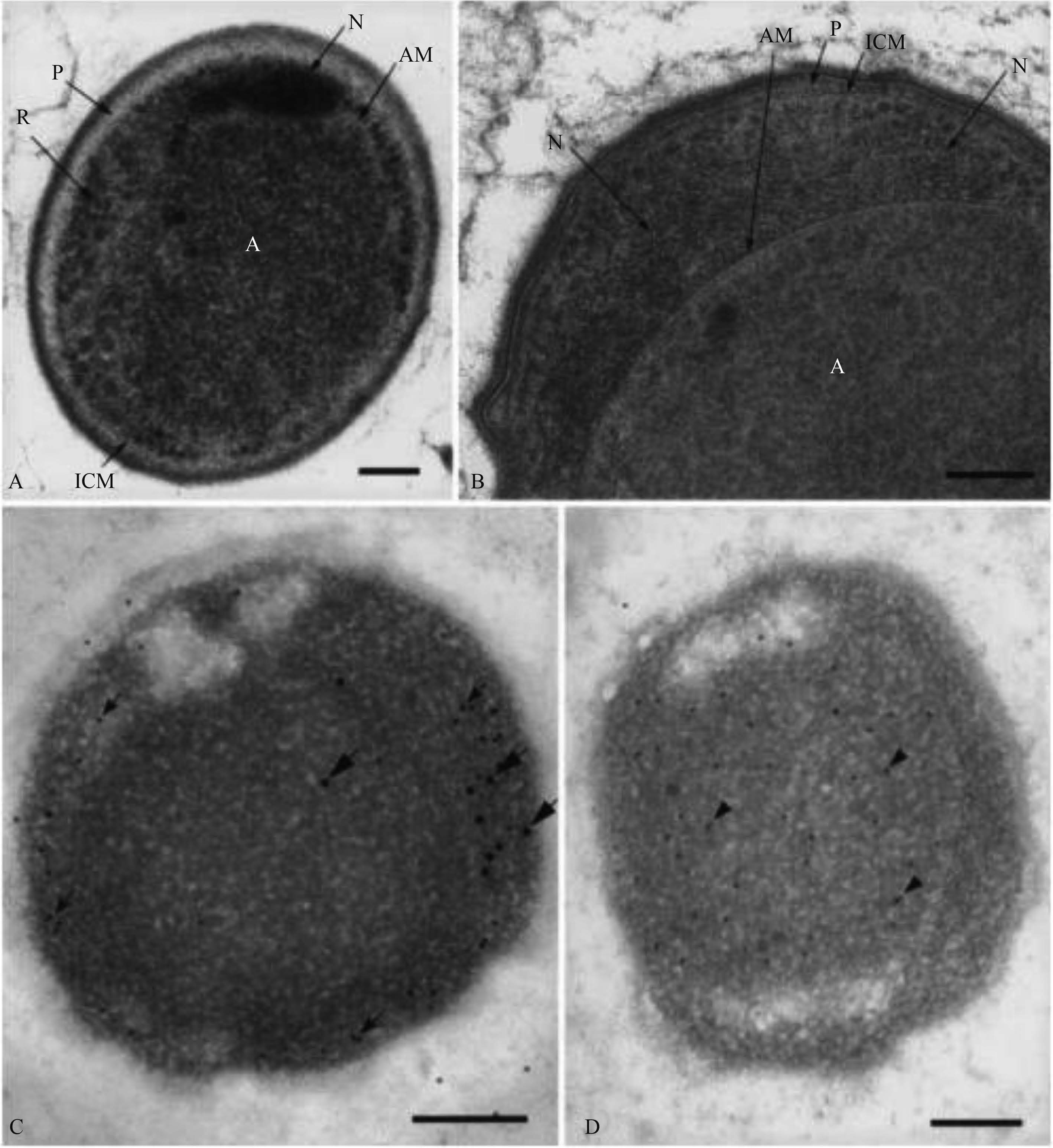
|
| 图3 中文Candidatus B. anammoxidans超薄切片[18] Figure3 A thin section of cryosubstituted cell of Candidatus B. anammoxidans[18]. The innermost compartment, the anammoxosome (A), filled with material of moderate electron density and granular texture, but devoid of ribosome-like particles, is surrounded by a single membrane (AM).The ribosome-containing riboplasm compartment (B) with nucleoid (N) attached to the anammoxosome membrane, and the paryphoplasm (P) bound on its inner side by a single intracytoplasmic membrane (ICM) surrounding the riboplasm. Thin section of cryosubstituted cell of Candidatus B. anammoxidans that has been labelled with anti-DNA antibody (C), visualized by secondary antibody conjugated to 15 nm colloidal gold (large arrows) and RNase conjugated to 10 nm colloidal gold (small arrows). Thin section of cryosubstituted cell of Candidatus B. anammoxidans labelled with anti-hydroxylamine oxidoreductase antibody (D) and visualised by secondary antibody conjugated to 10 nm colloidal gold (arrowheads). Hydroxylamine oxidoreductase is confined to the anammoxosome in the central region of the cell. |
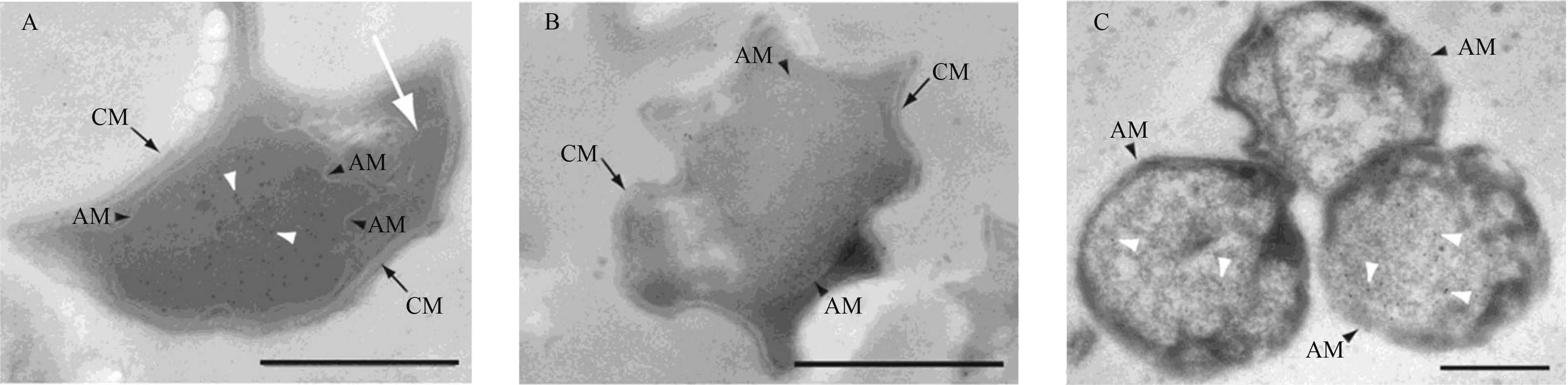
Candidatus Kuenenia stuttgartiensis电子断层成像模型的照片还显示,在厌氧氨氧化体膜旁,存在两个明显的结构:电子致密颗粒和管状结构(图4)。
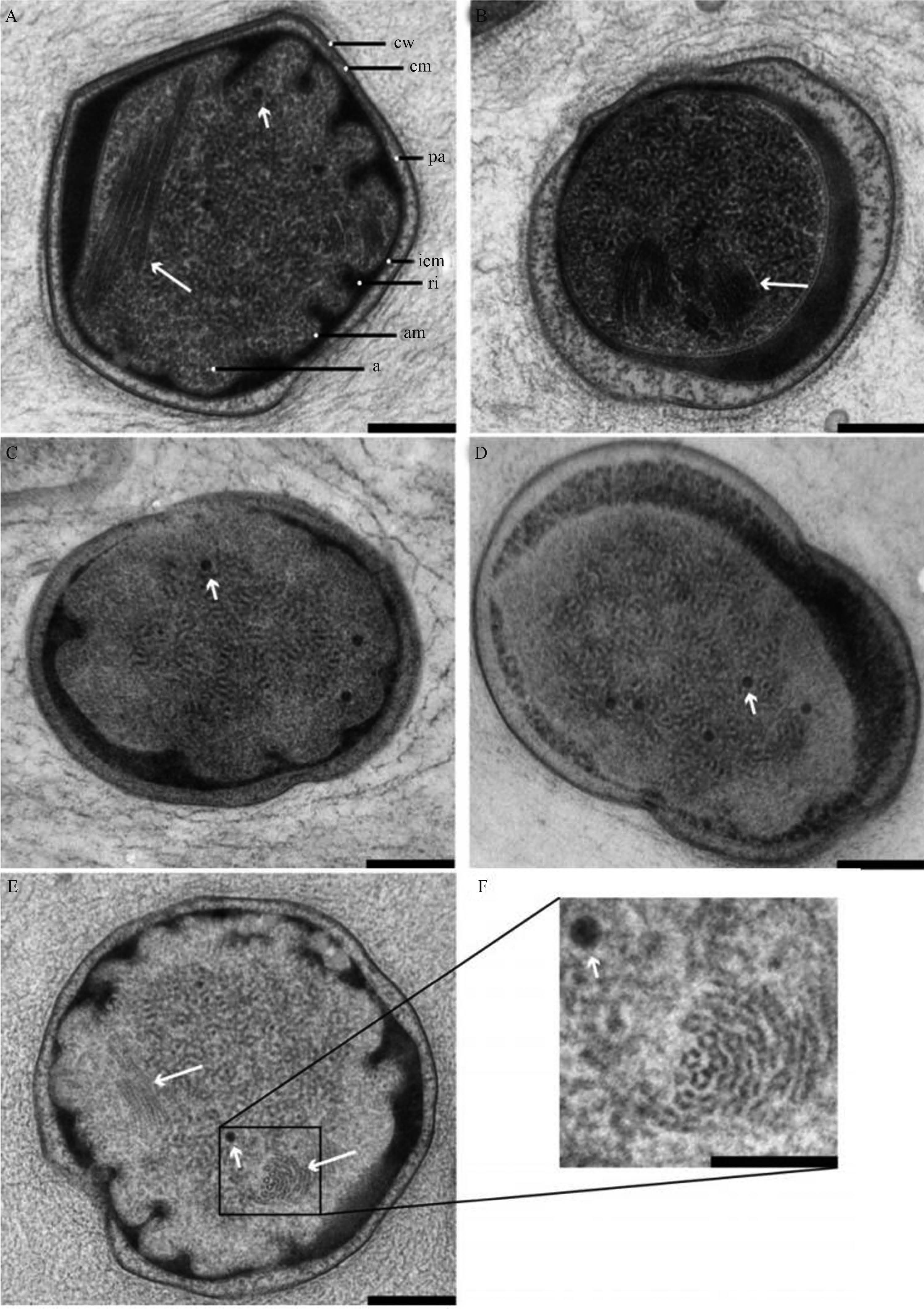
|
| 图4 AnAOB细胞的透射电子显微镜照片[19] Figure4 Transmission electron micrographs of cryofixed, freeze-substituted, and Epon-embedded anammox cells, showing the predominantly curved anammoxosome membrane, anammoxosome particles (smaller arrows) and tubule-like structures (larger arrows) [19]. Candidatus K. stuttgartiensis cells with curved (A) and oval (B) anammoxosome membrane; cw, cell wall; cm, cytoplasmic membrane; pa, paryphoplasm; icm, intracytoplasmic membrane; ri, riboplasm; am, anammoxosome membrane; a, anammoxosome. Candidatus B. fulgida cells with curved (C) and partly curved, partly oval (D) anammoxosome membrane. Candidatus K. stuttgartiensis cell showing transectioned tubule-like structures. Scale bars (A-E), 200 nm, (F), 100 nm. |
电子致密颗粒的直径为16至25 nm,在不同的厌氧氨氧化体中,其含量从1%到20%不等[5]。透射电镜-能量色散型X射线分析表明,电子致密颗粒含有铁、磷两种元素,据此推测它们是细菌铁蛋白。在Candidatus Kuenenia stuttgartiensis基因组中,发现2个基因(kuste3640和kuste4480)与大肠埃希氏菌铁蛋白Bfr有32%-48%的序列相似性。与不含血红素的细菌铁蛋白一样,含血红素的细菌铁蛋白也是铁储存蛋白。然而,目前对细菌铁蛋白的生理功能尚未研究清楚。
除了电子致密颗粒,在厌氧氨氧化体内还能观察到管状结构(图4-A,图4-B)。这些结构的横截面呈现六边形,由3个相同的单元构成。每个独立单元就是电子致密的六边形结构,平均宽度9.4 nm。这些单元合在一起构成长条状细管,有时呈堆积阵列,可以延展至厌氧氨氧化体的全长。据推测,这些小管样结构可能具有细胞骨架功能(在厌氧氨氧化体边界或膜弯曲处) [5]。
作为AnAOB的能量代谢中心,厌氧氨氧化体类似于线粒体(表2)。两者都形成褶皱以增加膜的表面积,提高膜两侧的物质传输能力。线粒体内膜含有大量的心磷脂,可调控线粒体内膜的物质传输;厌氧氨氧化体膜含有独特的梯形烷脂质,可抑制代谢中间产物逸散。两者的细胞都呈中等电子密度,内有电子致密颗粒,内含金属离子等。这意味着厌氧氨氧化菌的能量代谢与真核生物具有相似性。
| Object comparison | Anammoxsome | Mitochondrion |
| Coated membrane | Lipid bilayer | Lipid bilayer |
| Structure | Lipid bilayer, matrix, electron-dense particles | Outer membrane, intermembrane space, inner membrane, matrix, electron-dense particles |
| Matrix | Medium electron density | Medium electron density |
| Main lipids | Ladderane lipids | Phospholipids. Cardiolipin only in the inner membrane |
| Main function | Energy metabolism | Energy metabolism |
厌氧氨氧化的化学反应式(公式1)。
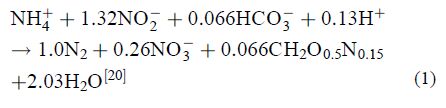
1997年,van de Graft等[21]基于15N示踪研究,提出了AnAOB代谢模型。其后,基于对AnAOB的基因组学和酶学研究,Strous等提出了改进模型[19, 22]。根据改进模型,亚硝酸先被含有细胞色素c和细胞色素d1(Cyt cd1)的亚硝酸还原酶(Nitrite reductase,Nir)转化成一氧化氮;再由联氨合成酶(HZS)将一氧化氮与氨转化成联氨;最后由联氨氧化酶(HZO)或羟氨氧化还原酶(HAO)将联氨转化成氮气。在联氨氧化成氮气的过程中,可产生4个电子,这4个电子通过Cyt c、泛醌、细胞色素bcl复合体以及其他细胞色素传递给Nir和HZS,其中3个电子传递给NiR,1个电子传递给HZS。伴随电子传递,质子被排放至厌氧氨氧化体膜外侧,在该膜两侧形成质子梯度,驱动ATP合成[23]。厌氧氨氧化代谢的主要功能酶有亚硝酸还原酶、联氨合成酶,联氨氧化酶,亚硝酸氧还酶,ATP合成酶等。各酶的功能与定位见表3。
| Enayme | Reaction | E0‘(V/e-) | Location |
| Nitrite reductase, Nir | NO2- + 2H+ + e- = NO + H2O | 0.38 | Periplasm |
| Hydrazine synthase, HZS | NH4++NO+2H++3e-=N2H4+H2O | 0.06 | Anammoxosome |
| Hyroxylamine/hydrazine oxidoreductase, HAO/HZO | N2H4=N2+4H++4e- | -0.75 | Anammoxosome |
| Nitrate/nitrite oxidoreductase, Nar | NO2-+H2O=NO3-+2H++2e- | -0.43 | Membrane |
Nir催化NO2-还原,基因组研究表明Candidatus K. stuttgartiensi中存在编码Cyt cd1型Nir的nirS基因[19]。但到目前为止,Nir尚未被分离纯化[23]。
2.2 联氨合成酶 (hydrazine synthase,HZS)HZS催化氨氮和NO生成联氨。在基因组中,HZS由8个蛋白基因编码,同一基因簇上还涉及到电子传递、分解代谢、几个细胞色素以及一个β折叠复杂体的编码基因。Karlsson等[25]利用HZS抗体蛋白kuste2860和kuste2861制成免疫胶体金进行标记,通过电镜观察确定了HZS的空间位置。研究表明,在AnAOB的厌氧氨氧化体内可见anti-kuste2860和anti-kuste2861抗体标记,而在厌氧氨氧化体外则未观察到抗体标记(图5)。这一研究结果证明HZS存在于厌氧氨氧化体内部。在厌氧氨氧化体内,抗体标记的颗粒呈有序排列,而非随机分布(图5-A)。
HAO和HZO是AnAOB中研究得较为深入的两种酶。HAO广泛存在于AOB和反硝化细菌中,它不但能够催化羟氨氧化成亚硝酸,也能够催化亚硝酸还原为羟氨,还能催化联氨氧化[23]。从AnAOB中分离获得的HAO不同于从AOB中获得的HAO,它不能将羟氨转化成亚硝酸,只能将其转化成NO或N2O[26]。HZO已从AnAOB中分离纯化,它只能催化联氨氧化,生成一分子氮气、4个质子和4个电子。HZO不能催化羟氨氧化,但羟氨能与该酶结合,从而对联氨产生竞争性抑制[27]。Lindsay等[18]通过免疫胶体金标记法,用HAO酶抗体和二次抗体共轭胶体金对Candidatus B. anammoxidans进行标记,发现HAO酶存在于厌氧氨氧化体内部 (图6-D)。
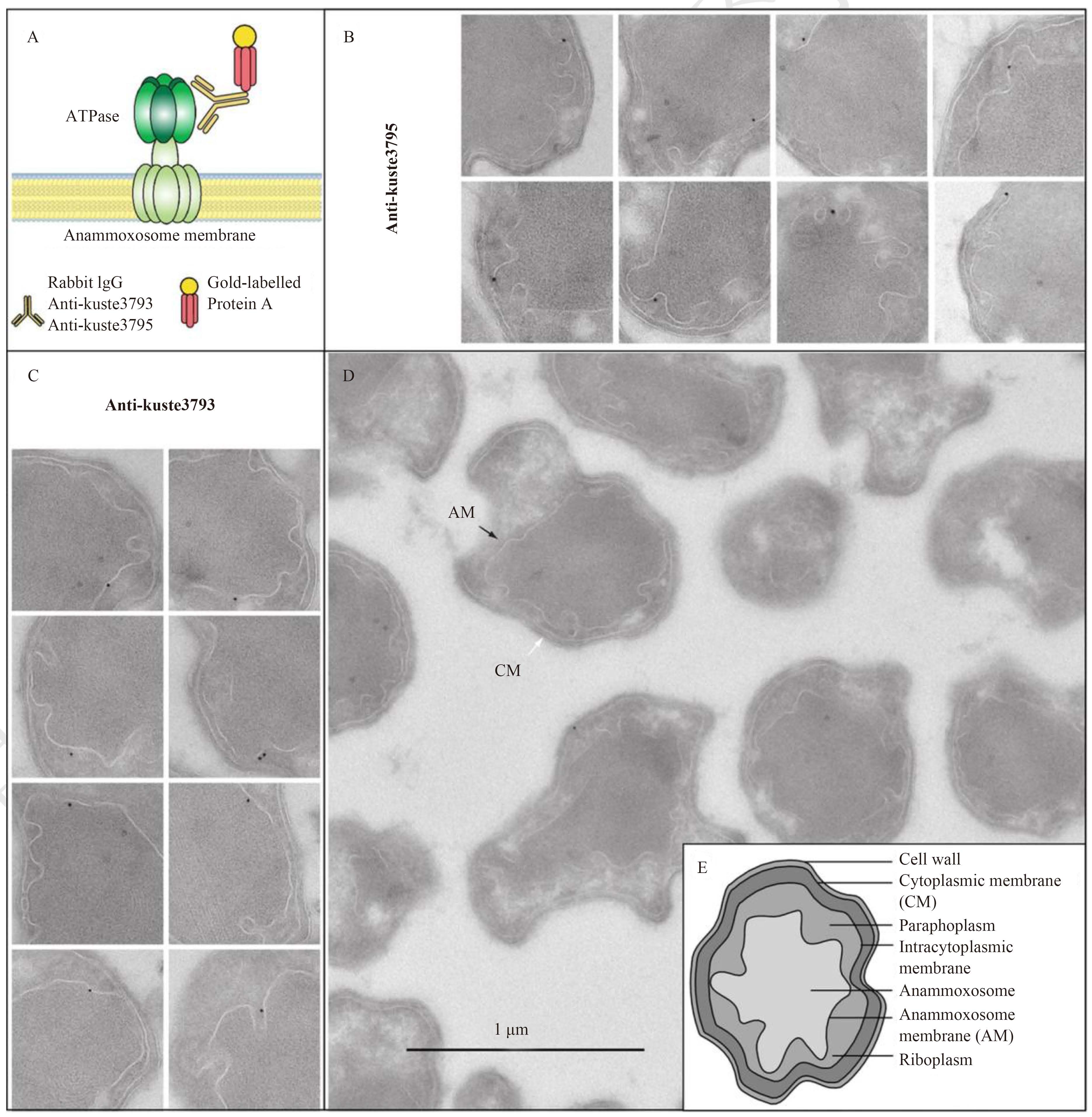
|
| 图6 F-ATP合成酶1的电镜免疫细胞化学和定位[29] Figure6 EM immunocytochemistry and localization of the F-ATPase 1[29]. A: Schematic illustration of the protocol for immunogold labeling. Target peptides from either the alpha (kuste3793)- or beta (kuste3795)-subunits of the identified F-ATPase 1 were chosen from the MS/MS analysis as linear epitopes. Antibodies against these peptides (anti-kuste3793 and anti-kuste3795) were raised in rabbits. During the immunogold localization, the antibodies bound to their target proteins in cryo-sectioned anammox colonies. Protein A labeled with gold particles (10 nm) was used to localize the antibodies, thereby revealing the location of the F-ATPase 1. B-C: Anammox cells incubated with the antibodies (anti-kuste3793 and anti-kuste3795). In each image, one or two 10 nm gold particles were associated with the anammoxosome membrane. Width of images is 500 nm. D: Colony of anammox bacteria after immunogold incubation with anti-CRAMP antibody (control). The cytoplasmic membrane (white arrow, CM) and the anammoxosome membrane (black arrow, AM) are indicated. E: Schematic illustration of the cellular organization of anammox bacteria[7]. |
基因分析表明,厌氧氨氧化菌中的Nar是一种硝酸盐-亚硝酸盐氧还酶。由于硝酸盐-亚硝酸盐氧还酶既具有氧化亚硝酸盐的功能性亚基,又具有将硝酸盐还原的功能性亚基[28],因此它是一个多功能酶,既可催化亚硝酸盐氧化,也可催化硝酸盐还原。
2.5 ATP合成酶 (ATP synthase,ATPase)厌氧氨氧化体膜上是否存在ATP酶,可用胶体金免疫电镜定位确认[5]。Candidatus Kuenenia stuttgartiensis基因组含有4个ATP酶基因簇:1个编码典型的F-ATP酶(F-ATP酶-1),2个编码非典型的F-ATP酶(F-ATP酶-2和-3),还有1个编码原核V-ATP酶(V-ATP酶-4)。采用催化亚基抗血清的免疫分析表明,F-ATP酶-1是最显著的膜结合ATP酶。免疫定位表明,F-ATP酶主要存在于厌氧氨氧化体膜的最内侧和厌氧氨氧化细胞膜的最外层。Karlsson等[29]通过ATP合成酶的抗体标记法,同样验证ATP合成酶与厌氧氨氧化体膜有关(图6)。
3 展望厌氧氨氧化是近年来微生物和环境领域的研究热点之一。AnAOB不仅是废水脱氮的重要功能菌,也是氮素循环的有力推动者,其特独的化学组成、细胞构造和代谢途径已成为微生物学研究的良好素材[30]。本文综述了AnAOB独特的细胞器——厌氧氨氧化体的组成、构造和功能。鉴于已有的研究基础和目前尚未解决的问题,未来的研究可从厌氧氨氧化体的力能学性状、酶学性状和细胞生物学性状等方面展开深入研究。
| [1] | Mulder A, van de Graaf AA, Robertson LA, Kuenen JG. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiology Ecology, 1995, 16(3):177-183. |
| [2] | Strous M, Fuerst JA, Kramer EHM, Logemann S, Muyzer G, van de Pas-Schoonen KT, Webb R, Kuenen JG, Jetten MSM. Missing lithotroph identified as new planctomycete. Nature, 1999, 400(6743):446-449. |
| [3] | Lu HF, Ding S, Zheng P. Central metabolism of anammox bacteria-A review. Acta Microbiologica Sinica, 2011, 51(8):1014-1022.(in Chinese)陆慧锋, 丁爽, 郑平. 厌氧氨氧化菌的中心代谢研究进展. 微生物学报, 2011, 51(8):1014-1022. |
| [4] | Dalsgaard T, Thamdrup B, Canfield DE. Anaerobic ammonium oxidation(anammox) in the marine environment. Research in Microbiology, 2005, 156(4):457-464. |
| [5] | van Niftrik L, Jetten MSM. Anaerobic ammonium-oxidizing bacteria:unique microorganisms with exceptional properties. Microbiology and Molecular Biology Reviews, 2012, 76(3):585-596. |
| [6] | Damsté JSS, Strous M, Rijpstra WIC, Hopmans EC, Geenevasen JA, van Duin AC, van Niftrik LA, Jetten MS. Linearly concatenated cyclobutane lipids form a dense bacterial membrane. Nature, 2002, 419(6908):708-712. |
| [7] | Kartal B, Rattray J, van Niftrik LA, van de Vossenberg J, Schmid MC, Webb RI, Schouten S, Fuerst JA, Damsté JS, Jetten MSM, Strous M. Candidatus "Anammoxoglobus propionicus" a new propionate oxidizing species of anaerobic ammonium oxidizing bacteria. Systematic and Applied Microbiology, 2007, 30(1):39-49. |
| [8] | Kartal B, Van Niftrik L, Rattray J, Van De Vossenberg JLCM, Schmid MC, Damsté JS, Jetten MSM, Strous M. Candidatus 'Brocadia fulgida':an autofluorescent anaerobic ammonium oxidizing bacterium. FEMS Microbiology Ecology, 2008, 63(1):46-55. |
| [9] | Kuypers MM, Sliekers AO, Lavik G, Schmid M, Jørgensen BB, Kuenen JG, Damsté JSS, Strous M, Jetten MS. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature, 2003, 422(6932):608-611. |
| [10] | Schmid M, Walsh K, Webb R, Rijpstra WI, van de PasSchoonen K, Verbruggen MJ, Hill T, Moffett B, Fuerst J, Schouten S, Damsté JSS, Harris J, Shaw P, Jetten M, Strous M.Candidatus "Scalindua brodae", sp. nov., Candidatus "Scalindua wagneri", sp. nov., two new species of anaerobic ammonium oxidizing bacteria. Systematic and Applied Microbiology, 2003, 26(4):529-538. |
| [11] | Jetten MSM, Sliekers O, Kuypers M, Dalsgaard T, Van Niftrik L, Cirpus I, Van de Pas-Schoonen K, Lavik G, Thamdrup B, Le Paslier D, Op den Camp HJM, Hulth S, Nielsen LP, Abma W, Third K, Engström P, Kuenen JG, Jørgensen BB, Canfield DE, Damsté JSS, Revsbech NP, Fuerst J, Weissenbach J, Wagner M, Schmidt I, Schmid M, Strous M. Anaerobic ammonium oxidation by marine and freshwater planctomycete-like bacteria. Applied Microbiology and Biotechnology, 2003, 63(2):107-114. |
| [12] | Rattray JE, van de Vossenberg J, Hopmans EC, Kartal B, van Niftrik L, Rijpstra WIC, Strous M, Jetten MSM, Schouten S, Damsté JSS. Ladderane lipid distribution in four genera of anammox bacteria. Archives of Microbiology, 2008, 190(1):51-66. |
| [13] | Damsté JSS, Rijpstra WIC, Schouten S, Fuerst JA, Jetten MSM, Strous M. The occurrence of hopanoids in planctomycetes:implications for the sedimentary biomarker record. Organic Geochemistry, 2004, 35(5):561-566. |
| [14] | Langworthy TA, Pond JL. Archaebacterial ether lipids and chemotaxonomy. Systematic and Applied Microbiology, 1986, 7(2):253-257. |
| [15] | Fuerst JA, Sagulenko E. Beyond the bacterium:planctomycetes challenge our concepts of microbial structure and function. Nature Reviews Microbiology, 2011, 9(6):403-413. |
| [16] | van Niftrik L, Geerts WJC, van Donselaar EG, Humbel BM, Yakushevska A, Verkleij AJ, Jetten MSM, Strous M. Combined structural and chemical analysis of the anammoxosome:a membrane-bounded intracytoplasmic compartment in anammox bacteria. Journal of Structural Biology, 2008, 161(3):401-410. |
| [17] | Kartal B, Maalcke WJ, de Almeida NM, Cirpus I, Gloerich J, Geerts W, Op den Camp HJM, Harhangi HR, Janssen-Megens EM, Francoijs KJ, Stunnenberg HG, Keltjens JT, Jetten MSM, Strous M. Molecular mechanism of anaerobic ammonium oxidation. Nature, 2011, 479(7371):127-130. |
| [18] | Lindsay MR, Webb RI, Strous M, Jetten MS, Butler MK, Forde RJ, Fuerst JA. Cell compartmentalisation in planctomycetes:novel types of structural organisation for the bacterial cell. Archives of Microbiology, 2001, 175(6):413-429. |
| [19] | Strous M, Pelletier E, Mangenot S, Rattei T, Lehner A, Taylor MW, Horn M, Daims H, Bartol-Mavel D, Wincker P, Barbe V, Fonknechten N, Vallenet D, Segurens B, Schenowitz-Truong C, Médigue C, Collingro A, Snel B, Dutilh BE, Op den Camp HJM, van der Drift C, Cirpus I, van de Pas-Schoonen KT, Harhangi HR, van Niftrik L, Schmid M, Keltjens J, van de Vossenberg J, Kartal B, Meier H, Frishman D, Huynen MA, Mewes HW, Weissenbach J, Jetten MSM, Wagner M, Le Paslier D. Deciphering the evolution and metabolism of an anammox bacterium from a community genome. Nature, 2006, 440(7085):790-794. |
| [20] | Strous M, Heijnen JJ, Kuenen JG, Jetten MSM. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Applied Microbiology and Biotechnology, 1998, 50(5):589-596. |
| [21] | Van De Graaf AA, De Bruijn P, Robertson LA, Jetten MSM, Kuenen JG. Metabolic pathway of anaerobic ammonium oxidation on the basis of 15N studies in a fluidized bed reactor. Microbiology, 1997, 143(7):2415-2421. |
| [22] | van Niftrik L, Geerts WJC, van Donselaar EG, Humbel BM, Webb RI, Fuerst JA, Verkleij AJ, Jetten MSM, Strous M. Linking ultrastructure and function in four genera of anaerobic ammonium-oxidizing bacteria:cell plan, glycogen storage, and localization of cytochrome C proteins. Journal of Bacteriology, 2008, 190(2):708-717. |
| [23] | Zheng P, Zhang L. Characterization and classification of anaerobic ammonium oxidation(anammox) bacteria. Journal of Zhejiang University(Agriculture & Life Science), 2009, 35(5):473-481.(in Chinese)郑平, 张蕾. 厌氧氨氧化菌的特性与分类. 浙江大学学报(农业与生命科学版), 2009, 35(5):473-481. |
| [24] | Jetten MSM, van Niftrik L, Strous M, Kartal B, Keltjens JT, Op den Camp HJM. Biochemistry and molecular biology of anammox bacteria. Critical Reviews in Biochemistry and Molecular Biology, 2009, 44(2/3):65-84. |
| [25] | Karlsson R, Karlsson A, Bäckman O, Johansson BR, Hulth S. Identification of key proteins involved in the anammox reaction. FEMS Microbiology Letters, 2009, 297(1):87-94. |
| [26] | Schalk J, de Vries S, Kuenen JG, Jetten MSM. Involvement of a novel hydroxylamine oxidoreductase in anaerobic ammonium oxidation. Biochemistry, 2000, 39(18):5405-5412. |
| [27] | Shimamura M, Nishiyama T, Shigetomo H, Toyomoto T, Kawahara Y, Furukawa K, Fujii T. Isolation of a multiheme protein with features of a hydrazine-oxidizing enzyme from an anaerobic ammonium-oxidizing enrichment culture. Applied and Environmental Microbiology, 2007, 73(4):1065-1072. |
| [28] | Zhang X, Lin WT, Zhu YN. Research progress of nitrite oxidoreductase in nitrobacteria. Microbilolgy, 2008, 35(11):1806-1810.(in Chinese)张星, 林炜铁, 朱雅楠. 硝化细菌中亚硝酸盐氧化还原酶的研究进展. 微生物学通报, 2008, 35(11):1806-1810. |
| [29] | Karlsson R, Karlsson A, Bäckman O, Johansson BR, Hulth S. Subcellular localization of an ATPase in anammox bacteria using proteomics and immunogold electron microscopy. FEMS Microbiology Letters, 2014, 354(1):10-18. |
| [30] | Kartal B, de Almeida NM, Maalcke WJ, Op den Camp HJM, Jetten MSM, Keltjens JT. How to make a living from anaerobic ammonium oxidation. FEMS Microbiology Reviews, 2013, 37(3):428-461. |
 2016, Vol. 56
2016, Vol. 56