
中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 赵成英, 刘海珊, 朱伟明
- Chengying Zhao, Haishan Liu, Weiming Zhu
- 海洋曲霉来源的新天然产物
- New natural products from the marine-derived Aspergillus fungi-A review
- 微生物学报, 2016, 56(3): 331-362
- Acta Microbiologica Sinica, 2016, 56(3): 331-362
-
文章历史
- 收稿日期:2015-10-15
- 修回日期:2015-12-25
- 网络出版日期:2015-12-30
海洋真菌由于其遗传背景复杂、代谢产物种类多、产量高,成为海洋微生物新天然产物的主要来源,作者对2010-2013年初的海洋微生物来源新天然产物的统计表明,研究最多的真菌属是曲霉(Aspergillus),占海洋真菌新天然产物的31%[1]。海洋曲霉源天然产物的研究始于1992年,Shinggu等报道了首例海洋曲霉来源的新天然产物fumiquinazolines A-C (149-151)[2];截止到2014年8月,已报道512个海洋曲霉来源的新天然产物。其结构类型多样(包括聚酮、生物碱、萜类、甾体、卤代物、脂肪酸、肽类、糖苷等),且有多种生物活性(包括抗癌、抑菌、自由基清除和抗寄生虫等)。值得一提的是,由海洋曲霉天然产物halimide(301)[3, 4]衍生而来的plinabulin (NPI-2358)[5]是一种血管阻断剂,作用于肿瘤细胞,影响微管蛋白解聚;目前plinabulin已结束II期临床研究、开始在美国和中国进行III期临床研究,用于治疗转移性的晚期非小细胞肺癌(NSCLC)[6],成为20个海洋药物之一,也是唯一的海洋曲霉菌来源的药物[7]。由此可见海洋曲霉是海洋天然产物乃至新药发现的重要资源,本文将综述这些海洋曲霉新天然产物的菌株来源、结构及其生物活性。
1 海洋动物来源的曲霉天然产物 1.1 海绵来源的曲霉天然产物氯代物chlorocarolides A(1)和B(2)来源于赫曲霉Aspergillus cf.ochraceus 941026[8]。1株黑曲霉Aspergillus niger代谢产生二酮哌嗪(环缩二氨酸)的二聚体asperazine(3),该化合物可以选择性抑制人白血病细胞L1210、C38以及人结肠癌细胞H116或者CX1细胞株[9]。化合物asperic acid(4)是从另外1株黑曲霉A. niger 94-1212的次生代谢产物中分离得到[10]。花斑曲霉A. versicolor (Vuill) Triab代谢产生六元色酮的衍生物aspergiones A-F(5-10)[11]以及aspergillone(11)、aspergillodiol(12)、aspergillol(13)和12-acetyl-aspergillol(14)[12]。3个含氯的抗生素15-17来自孔曲霉A. ostianus TUF 01F313,均对大西洋鲁杰氏菌Ruegeria atlantica有抑制活性,其中化合物16和17在25 μg/disc浓度下的抑菌圈直径分别为10.1 mm和10.5 mm,而化合物15在5 μg/disc时即表现出较好的抑制活性(抑菌圈直径为12.7 mm),另外该化合物还有微弱的金黄色葡萄球菌抑制活性(25 μg/disc时抑菌圈直径为10.2 mm)[13]。进一步研究还分离到aspinotriols A(18)和B(19)、aspinonediol(20)[14],aspergillides A-C(22-24)[15]及其七环生物碱化合物21-hydroxystephacidin A(25)[16](图 1)。并确定了dihydroaspyrone(21)的绝对构型[14],其中化合物22-24对L1210细胞的LD50分别为2.1、71.0和2.0 μg/mL[15]。
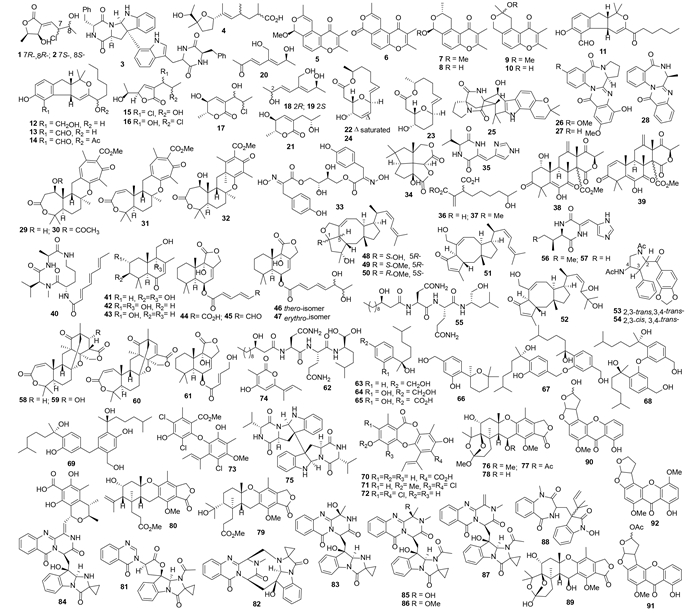
|
| 图1 化合物1–92的结构 Figure 1. Structures of compounds 1–92. |
Circumdatins D-F(26-28)分离自另外一株孔曲霉A. ostianus IBT 12704[17]。混源萜tropolactones A-D(29-32)由曲霉属Aspergillus sp. CNK-371代谢产生,其中化合物29-31对HCT-116细胞有微弱的抑制活性,IC50分别为 13.2、10.9 和13.9 μg/mL[18]。棘孢曲霉A. aculeatus CRI323-04代谢产生aspergillusol A(33)[19]和asperaculin A(34)[20],其中化合物33对酵母和嗜热脂肪芽孢杆菌来源的α-糖苷酶有抑制活性,IC50值分别为465和1060 μmol/L[19]。化合物35-37来自另一株棘孢曲霉A. aculeatus CRI322-03[21]。曲霉A. insuetus代谢产生Terretonins E(38)和F(39),均具有哺乳动物线粒体呼吸链抑制作用,IC50值分别为3.90和2.97 μmol/L[22]。菌核曲霉A. sclerotiorum Huber SP080903f04代谢产生N-去甲棕曲菌素JBIR-15(40)[23]。焦曲霉A. ustus 8009代谢产生7个补身烷倍半萜化合物41-47,其中44、45对多种肿瘤细胞有细胞毒活性,尤其是45对L5178Y细胞株的EC50值为0.6 μg/mL[24]。蛇孢甲壳素48-52及吡咯生物碱53、54也来自同一株菌[25]。脂肽fellutamide C(55)(图 1)来自于A. versicolor,对SK-MEL-2、XF498和HCT15的IC50分别为5.1、3.9和3.1 μmol/L[26]。
JBIR-74(56)和JBIR-75(57)来自Aspergillus sp. fs14[27]。混源萜insuetolides A-C(58-60)和补身烷倍半萜61来自奇突曲霉A. insuetus OY-207,其中化合物58有抑制粗糙链孢霉菌的活性、MIC为140 μmol/L,化合物60和61对人MOLT-4细胞有细胞毒活性、50 mg/mL时的抑制率分别为51%和55%[28]。脂肽fellutamide F(62)来自A. versicolor PF10M,对多株人癌细胞的EC50为0.13-1.81 μg/mL[29]。Aspergillus sp.代谢产生4个没药烷倍半萜aspergiterpenoid A(63)、(-)-sydonol(64)、(-)-sydonic acid(65)和化合物66,其对金黄色葡萄球菌、枯草芽孢杆菌、蜡状芽孢杆菌等有抑菌活性(MIC为1.25-20.0 μmol/L)[30]。没药烷倍半萜二聚体disydonols A-C(67-69)也来自同一株菌,其中化合物67和69对HepG-2和Cashi细胞均有细胞毒活性(IC50分别为9.31/12.40 μg/mL和2.91/10.20 μg/mL)[31]。缩酚酸环醚70-72、二芳基醚73及吡喃酮74来自爪甲曲霉A. unguis CRI282-03,其中化合物70-72均有芳香酶抑制活性(IC50分别为2.2、4.1和0.7 μmol/L),且70和71在黄嘌呤氧化测试中表现出自由基清除活性(IC50分别为16.0和<15.6 μmol/L)[32]。二酮哌嗪二聚体eurocristatine(75)产自冠突散囊菌Eurotium cristatum KUFC 7356[33],E. cristatum是小冠曲霉A. cristatellus的有性型。1株Aspergillus sp.代谢产生austalides M-Q(76-80)[34]、tryptoquivaline K (81)和 fumiquinazolines K-P (82-87)[35]以及生物碱88和混源萜austalide R(89)[36] (图 1)。其中化合物81在10 μg/mL浓度下对小鼠淋巴瘤细胞L5178Y的抑制率为23%[35],化合物88能选择性抑制弧菌(Vibrio sp.)生长,而89则广谱抗菌、其对Vibrioharveyi、V. proteolyticus、V. carchariae、Shewanella putrefaciens、Roseobacter litoralis、Pseudoalteromonas elyakovii、P. irgensii和Halomonas aquamarina均有抑制作用[36]。
杂色曲霉A. versicolor MF359代谢产生3个柄曲霉素90-92(图 1),其中化合物92对金黄色葡萄球菌和枯草芽孢杆菌均有抑制活性,MIC分别为12.500 μg/mL和3.125 μg/mL[37]。黑曲霉A. niger代谢产生3,3-bicoumarin bicoumanigrin(93)、aspernigrins A和B(94、95)以及pyranonigrins A-D(96-99)(图 2);化合物93在浓度1-20 μg/mL时具有人肿瘤细胞毒活性,化合物94、95在浓度为50 μg/mL有轻微的肿瘤细胞毒活性;化合物95具有神经保护作用[38]。Nafuredin(100)产自黑曲霉Aspergillus niger FT-0554,表现出猪蛔虫Ascarissuum延胡索酸还原酶(NFRD)抑制活性,其IC50为12 nmol/L[39]。
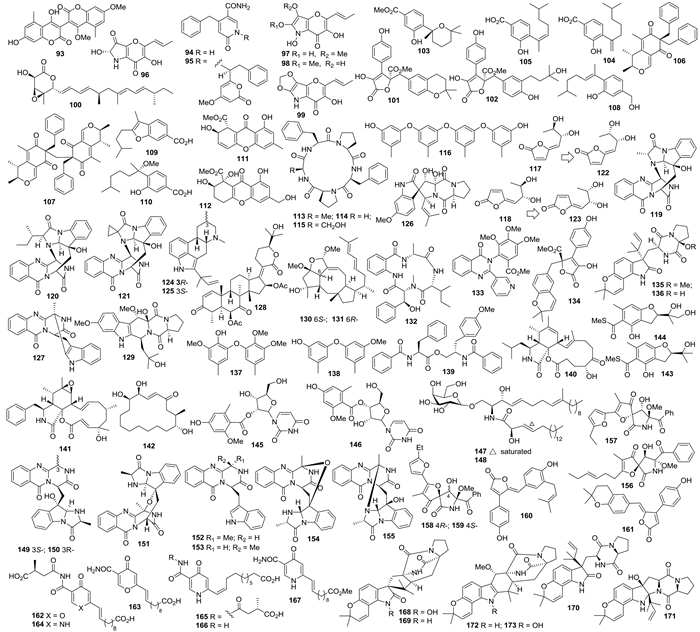
|
| 图2 化合物93–173的结构 Figure 2. Structures of compounds 93–173. |
土曲霉A. terreusHKI0499代谢产生aspernolides A、B(101、102)[40]。(+)-methyl sydowate(103)、7-deoxy-7,14-didehydrosydonic acid(104)和7-deoxy-7,8-didehydrosydonic acid(105)(图 2)来自一株Aspergillus sp.,其中化合物103可抑制金黄色葡萄球菌、100 μg/mL浓度下抑菌圈直径为11 mm[41]。Aspergilones A、B(106、107)来自同一株菌,其中化合物106对HL-60、MCF-7和A549细胞的IC50分别为3.2、25.0和27.0 μg/mL,且有抗污损作用、EC50为7.7 μg/mL[42]。A. sydowii PSU-F154代谢产生倍半萜aspergillusenes A (108)、B (109)和methylsydonic acid (110),以及氧杂蒽酮aspergillusones A(111)和B(112)[43]。A. versicolor LCJ-5-4代谢产生环五肽versicoloritides A-C(113-115)、地衣酚四聚体tetraorcinol A(116)、内酯versicolactones A、B(117、118)[44]以及喹唑啉酮生物碱cottoquinazolines B-D(119-121)[45],其中化合物116有弱的DPPH自由基清除活性(IC50为67 μmol/L),化合物121对白色念珠菌有中等抑菌活性(MIC 22.6 μmol/L);化合物116[46]、121[47]被NPR选为热点化合物;经过全合成研究,化合物versicolactones A和B的结构分别修正为122和123[48]。烟曲霉A. fumigates代谢产生新吲哚生物碱124和125[49]。二酮哌嗪生物碱spirotryprostatin F(126)[50]和fumiquinazoline K(127)以及萜类化合物128[51]来自另一株烟曲霉A. fumigatus KMM 4631,其中化合物126在较低浓度(0.1-1.0 μmol/L)时可以促进大豆、荞麦和小麦发芽[50]。吲哚生物碱cyclotryprostatin E(129)来自萨氏曲霉A. sydowii SCSIO 00305[52]。内生曲霉Aspergillus sp.代谢产生二倍半萜ophiobolin O(130)和6-epi-ophiobolin O(131),对P388均有较强的细胞毒活性,IC50分别为4.7和9.3 μmol/L[53],且化合物130可以使MCF-7周期停滞于G0/G1期(IC50为17.86 μmol/L)、并通过MAPK途径使MCF-7呈现时间和剂量依赖的细胞凋亡[54]。土曲霉菌A. terreus SCSGAF0162代谢产生asperterrestide A(132)、terremide C(133)和aspernolide E(134)(图 2),化合物132对组织细胞淋巴瘤细胞U937和急性淋巴母细胞白血病细胞MOLT-4的IC50值分别是6.4和6.2 μmol/L,对流感病毒H1N1和H3N2的IC50值分别是15.0 μmol/L和8.1 μmol/L[55]。
曲霉菌Aspergillus sp. XS-20090066代谢产生吲哚生物碱17-epi-notoamidesQ、M(135、136)和苯醚衍生物cordyols D、E(137、138)[56]。曲霉菌A. elegansZJ-2008010代谢产生4′-OMe-asperphenamate(139)和细胞松弛素aspochalasins A1和Z24(140、141),其中化合物139对表皮葡萄球菌S. epidermidis表现出了选择性的抑制活性(MIC值为10 μmol/L)[57]。曲霉菌Aspergillus sp. SCSGAF 0076代谢产生了大环内酯aspergillide D(142)[58]。赤散囊菌Eurotium rubrum SH-823代谢产生硫代物eurothiocins A和B(143和144),抑制α-糖苷酶的IC50值分别为17.1 μmol/L和42.6 μmol/L[59]。杂色曲霉A. versicolor代谢产生核苷类化合物145和146,对表皮葡萄球菌有选择性抑制作用、MIC值为12.5 μmol/L,对卤虫有致死活性,LC50值为8.4 μg/mL[60]。曲霉菌A. flavipes代谢产生脑苷脂flavicerebrosides A和B(147和148)(图 2),均对KB细胞有细胞毒活性,IC50值分别为20.7 μg/mL和14.3 μg/mL[61]。
1.3 其它动物来源的曲霉天然产物Fumiquinazolines A-C(149-151)来自烟曲霉A. fumigatus OUPS-T106B-5,有中等细胞毒活性[2](Numata et al. 1992),其结构通过全合成确证[62, 63];该菌株还产生fumiquinazolines D-G(152-155)[64]、cephalimysin A(156)[65]及cephalimysins B-D(157-159)[66],其中化合物149、150及152-155对P388细胞有细胞毒活性,ED50分别为6.1、16.0、13.5、13.8、14.6和17.7 μg/mL[64],化合物156对P388和HL-60细胞的IC50值分别为15.0 nmol/L和9.5 nmol/L[65],化合物158和159对HL-60的IC50分别为58.4和48.7 μmol/L[66]。曲霉A. terreus OUCMDZ-1925代谢产生rubrolides R(160)和S(161),抑制K562细胞的IC50分别为12.8和10.9 μmol/L,化合物161还具有抗H1N1病毒的活性、IC50为87.1 μmol/L,化合物160具有ABTS和DPPH自由基清除活性,IC50分别为1.33 mmol/L和43.4 μmol/L[67]。曲霉Aspergillus sp. MF275代谢产生himeic acids A-C(162-164)[68]和himeic acids E-G(165-167)[69] (图 2),其中化合物162具有泛素激活酶E1抑制活性,50 μmol/L时的抑制率为65%[68]。
曲霉Aspergillus sp. MF297-2代谢产生notoamides A-D(168-171)(图 2),化合物168-170对Hela及L1210细胞的IC50值为22-52 μg/mL[70];该菌株还代谢产生notoamides F-K(172-177)(图 3),其中175对Hela有弱活性、IC50为21 μg/mL[71]。随后,又从该菌株的代谢产物中相继分离鉴定了notoamide E(178)和notoamides E2-E4(179-181)[72]、(-)-versicolamide B(182)和notoamides L-N(183-185)[73]、notoamides O-R(186-189)[74]以及notoamide S(190)[75]。具有倍半萜母核的吡啶生物碱pileotin A(191)则来自烟曲霉A. fumigatus OUPS-N138[76]。变色曲霉A. versicolor OUPS-N136代谢产生吲哚二萜anthcolorins A-F(192-197)(图 3),其中193-195对P388细胞的IC50值在2.2-8.5 μmol/L之间[77]。
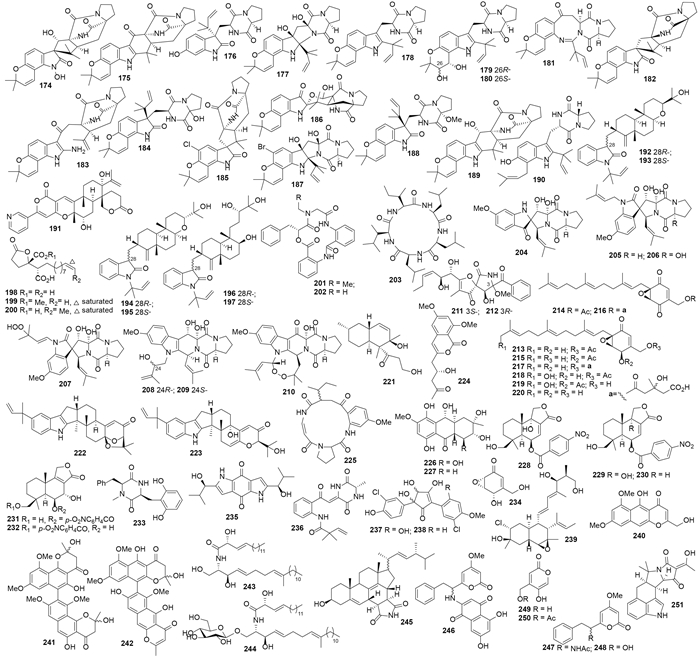
|
| 图3 化合物174–251的结构 Figure 3. Structures of compounds 174–251. |
Spiculisporic acids B-D(198-200)来自内生曲霉Aspergillus sp. HDf2[78]。环肽clavatustides A、B(201、202)[79]和C(203)[80]来自1株棒曲霉A. clavatus C2WU,其中201和202呈现剂量依赖的肝癌细胞系(HCC)增殖抑制活性。此外,201、202还可将人肝癌细胞HepG-2的细胞周期阻滞在G1期[79]。化合物204、spirotryprostatins C-E(205-207)、fumitremorgin B的衍生物(208、209)和13-oxoverruculogen(210)来自一株烟曲霉 A. fumigatus,均有细胞毒活性,尤其是化合物207对MOLT-4、HL-60和A549细胞的IC50分别为3.1、2.3、3.1 μmol/L,208对HL-60、BEL-7402细胞的IC50分别为3.4、7.0 μmol/L,209和210对HL-60细胞的IC50分别为5.4 μmol/L和1.9 μmol/L[81]。另一株烟曲霉A. fumigatus WFZ-25代谢产生螺内酰胺pseurotins A1和A2(211和212)[82]。Yanuthones A-E(A213-A217)、1-hydroxyyanuthones A、C(218、219)及22-deacetylyanuthone A(220)(图 3)产自黑曲霉A. niger F97S11[83]。
曲霉菌Aspergillus sp. MF297代谢产生多聚乙酰aspermytin A(221),在50 μmol/L时221可诱导小鼠嗜铬细胞瘤PC-12细胞神经轴突生长[84]。黄曲霉A. flavus OUCMDZ-2205代谢产生吲哚二萜222、223和异香豆素224,其中化合物222对金黄色葡萄菌的MIC值为20.5 μmol/L,在10 μmol/L时222和223可将A549细胞阻滞在S期,化合物222对PKC-β的IC50值为15.6 μmol/L[85]。柄曲霉A. flavipes Z-4代谢产生环肽225[86](图 3)。
2 植物来源的曲霉天然产物 2.1 海藻来源的曲霉天然产物Tetrahydrobostrycin(226)和1-deoxytetrahydrobostrycin(227)来自曲霉Aspergillus sp. 05F16[87]。倍半萜insulicolide A(228)来自胰岛曲霉A. insulicola[88]。杂色曲霉A. versicolor CNC 327代谢产生化合物229-232(图 3),其中229表现出较强细胞毒活性,对HCC-2998、HCT-116、BT-549和SNB-75细胞的LC50分别为0.53、0.44、0.27和0.44 μg/mL,其对5株肾肿瘤细胞(786-0、ACHN、CAK-1、TK-10和UO-31)表现出选择性抑制活性、LC50约为0.47-0.57 μg/mL[89]。曲霉Aspergillu sp.代谢产生mactanamide(233),具有抑制白色念珠菌活性[90]。Parasitenone(234)来自一株曲霉A. parasiticus MFA153[91]。手性双吡咯并蒽terreusinone(235)产自土曲霉A. terreus MFA 460,具有防护紫外线A的活性,ED50值为70 μmol/L[92]。曲霉菌Aspergillus sp. MFA 212代谢产生二酮哌嗪golmaenone(236),具有防护紫外线A的活性(ED50值为90 μmol/L)和DPPH自由基清除活性,IC50值为20 μmol/L[93]。Sydowins A、B(237、238)来自萨氏曲霉A. sydowii[94]。从曲霉Aspergillus sp. MFB024的代谢产物中发现1个多氧萘氢衍生物239(chlorofusarielin B),其对金黄色葡萄球菌、甲氧西林耐药葡萄球菌、多药耐药葡萄球菌具有一定的抑制作用,其MIC均为62.5 μg/mL [95]。Nigerasperones A-C(240-242)为黑曲霉A. niger EN-13的产物,化合物242对白色念珠菌的抑菌圈为9 mm(两性霉素的抑菌圈为12 mm)、对DPPH自由基的清除率为41.6%[96]。此菌还代谢产生asperamides A和B(243和244)[97]、ergosterimide(245)[98]、246[99]、isopyrophen(247)和aspergillusol(248)[100],其中化合物243对白色念珠菌的抑菌圈为12 mm[97]。
化合物249、250[101]和iso-α-CPA(251)(图 3)[102]产自黄曲霉A. ?avusc-f-3,化合物251对A549细胞的IC50值为42.2 μmol/L[102]。赭曲霉A. ochraceus EN-31代谢产生2-hydroxycircumdatin C(252)[103]、7-nor-ergosterolide(253)和化合物254、255[104](图 4),化合物252具有明显的DPPH自由基清除作用,其IC50值为9.9 μmol/L[103]。吲哚二萜asporyzin A-C(256-258)[105]和甾类asporyergosterol(259)[106]来自米曲霉A. oryzaercf-2,其中化合物A258有较强的大肠杆菌抑制作用(每孔30 μg给药时,抑菌圈为8 mm)[105]。JBIR-81(260)和JBIR-82(261)来自曲霉Aspergillus sp. SpD081030G1f1,是有效的自由基清除剂(对N18-RE-105细胞的L-谷氨酸毒性的EC50分别为0.7和1.5 μmol/L,强于对照组的8.8 μmol/L)[107]。脑苷脂类flavusides A(262)和B(263)来自黄曲霉A. flavus,抑制金黄色葡萄球菌的MIC为15.6 μg/mL,对MRSA的MIC约为31.2 μg/mL[108]。脂肪酸264和甾体265来自另一株黄曲霉A. flavus cf-5[109]。肉色曲霉A. carneus KMM4638代谢产生吲哚生物碱carneamides A-C(266-268)、喹唑酮生物碱carnequinazolines A-C(269-271)、芳基糖苷carnemycins A、B(272、273)及倍半萜274[110]。喹唑啉酮衍生物275、276和二苯醚衍生物277也来自该菌[111]。二萜asperolides A-C(278-280)[112]、wentiquinone C(281)和282[113] (图 4)来自温特曲霉A. wentii EN-48,其中化合物278和279对多种肿瘤细胞有弱的细胞毒活性(IC50为35-97 μmol/L)、化合物282有明显的DPPH自由基清除活性(IC50为5.2 μg/mL)。
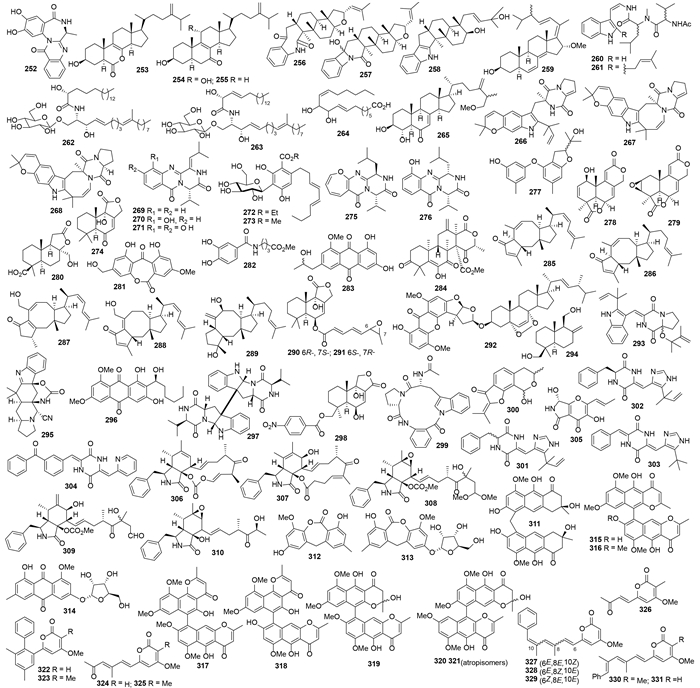
|
| 图4 化合物252–331的结构 Figure 4. Structures of compounds 252–331. |
化合物283来自一株变色曲霉A. versicolor,对枯草芽孢杆菌、蜡状芽孢杆菌和金黄色葡萄球菌有中等强度的抑菌活性(每孔100 μg给药时的抑菌圈分别为11、12和14 mm)[114]。萜类化合物1,2-dihydroterretonin F(284)、(6α)-21-deoxyophiobolin G(285)、(6α)-16,17-dihydro-21-deoxyophiobolin G(286)、ophiobolin U(287)、ophiobolin V(288)、ophiobolinW(289)和290、291(图 4)来自焦曲霉A. ustus cf42,其中化合物287对大肠杆菌和金黄色葡萄球菌均有抑菌活性、每孔30 μg给药时的抑菌圈分别为15和10 mm[115]。Asperversin A(292)和293产自变色曲霉A. versicolor pt20[116]。花斑曲霉A. versicolor dl29代谢产生倍半萜albican-11,14-diol(294)[117]和生物碱aspeverin(295)[118],其中化合物294对卤虫的LC50为35.0 μg/mL,且为大肠杆菌和金黄色葡萄球菌的抑制剂、30 μg/孔时的抑菌圈分别为7和10.3 mm[117],而化合物295对赤潮异弯藻Heterosigma akashiwo的EC50值分别为6.3 μg/mL(24h)和3.4 μg/mL(96h)[118]。蒽醌6,8-di-O-methylaverantin(296)来自变色曲霉A. versicolor EN-7[119]。蜡叶散囊菌E. herbariorum HT-2代谢产生了吲哚二酮哌嗪二聚体cristatumin E(297),其对K562细胞的IC50值为8.3 μmol/L,对产气杆菌Enterobacter aerogenes和大肠杆菌Escherichia coli的MIC值分别为44.0和44.0 μmol/L[120]。赭曲霉A. ochraceus Jcma1F17代谢产生倍半萜6β,9α-dihydroxy-14-p-nitrobenzoylcinnamolide(298),对10种人体肿瘤细胞(H1975、U937、K562、BGC-823、Molt-4、MCF-7、A549、Hela、HL60和Huh-7)的IC50为1.95-6.12 μmol/L,对H3N2病毒和EV71病毒的IC50分别为17.0和9.4 μmol/L[121]。2株曲霉菌Aspergillus sp. BM-05和BM-05ML共培养产生环三肽psychrophilin E(299)(图 4),对HCT-116细胞的IC50值为28.5 μmol/L[122]。曲霉菌A. pseudodeflectus代谢产生pseudodeflectusin(300),对NUGC-3、HeLA-S3和HL-60均有细胞毒活性,其中对HL-60细胞的IC50值为39 μmol/L[123]。
1997年,Fenical等从曲霉Aspergillus sp. CNC139的代谢产物中分离得到二酮哌嗪类halimide(301),其对HCT116和A2780细胞有较强的细胞毒活性,IC50分别为1 μmol/L和0.8 μmol/L[3, 4]。同时Kanoh等也从焦曲霉A. ustus NSC-F038的代谢产物中分离得到,将其命名为phenylahistin,并发现(-)-phenylahistin(302)才是真正的活性成分,对P388的IC50为0.35 μmol/L,而且1 μmol/L时可以将该细胞阻滞在G2/M期[124],良好的生物活性使其成为先导结构。之后多个课题组通过全合成出[125, 126],构效关系研究筛选出plinabulin(NPI-2358)(303)[5],作为肿瘤细胞的血管分裂剂进入了Ⅱ期临床研究[127, 128, 129]。目前,plinabulin已经结束其II期临床研究,并于2015年第三季度开始在美国和中国进行其III期临床研究,用于治疗转移性的晚期非小细胞肺癌[6]。Gerwick所列的20个海洋药物中(包括7个上市药和13个临床药物),303是唯一的海洋曲霉属真菌来源的药物[7]。近期,Hayashi小组对plinabulin进行结构改造得到活性更好的化合物KPU-300(304)(图 4),304对HT-29细胞的IC50为7.0 nmol/L,可以有效的与微管蛋白结合(Kd = 1.3 μmol/L),诱导微管解聚[130]。
2.2 红树林来源的曲霉天然产物黑曲霉A. niger LL-LV3020代谢产生pyranonigrin A(305)[131]。黄柄曲霉A. favipes代谢产生cytochalasins Z16-Z20(306-310)[132]。赤散囊菌Eurotium rubrum QEN-0407-G2代谢产生蒽酮衍生物eurorubrin(311)和312-314,其中化合物311显示了中等强度的DPPH自由基清除活性,IC50值为44.0 μmol/L[133]。苯并-γ-吡喃酮二聚体rubasperones A-C (315-317)[134]和rubasperones D-E(318-321)[135]分离自塔宾曲霉A. tubingensis GX1-5E。Nigerapyrones A-H(322-329)和已知物asnipyrones A和B来自黑曲霉A. niger MA132,其中已知化合物asnipyrones A和B的结构分别被修正为330和331(图 4);化合物323对HepG2细胞的IC50为62 μmol/L,326对SW1990、DMA-MB-231和A549细胞的IC50分别为38、48和43 μmol/L[136]。单萜acetoxydehydroaustin B(332)和1,2-dihydroacetoxydehydroaustin B(333)(图 5)来自Aspergillus sp. 085241B[137]。黄曲霉A. flavus 092008代谢产生黄曲霉毒素aflatoxin B2b(334),其对大肠杆菌、枯草芽孢杆菌和产气杆菌有中等的抑菌活性(MIC分别为22.5、1.7和1.1 μmol/L),对A549、K562和L-02细胞的IC50分别为8.1、2.0和4.2 μmol/L[138]。Aspergillumarins A(335)和B(336)产自曲霉Aspergillus sp.,在50 μg/mL时对金黄色葡萄球菌和芽孢杆菌有抑制活性[139]。
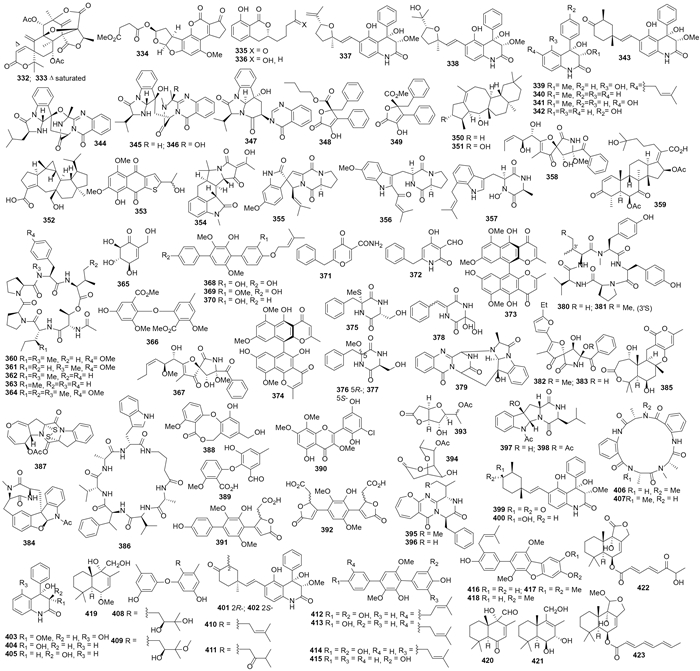
|
| 图5 化合物332–423的结构 Figure 5. Structures of compounds 332–423. |
构巢曲霉A. nidulans MA-143代谢产生aniduqui-nolones A-C(337-339)、6-deoxyaflaquinolone E(340)、isoaflaquinolone E(341)、14-hydroxyaflaquinolone F(342)和已知物aflaquinolone A(343)(图 5);化合物338、339和343具有抗卤虫活性,LD50值分别是7.1、4.5和5.5 μmol/L[140]。喹唑啉酮生物碱aniquinazolines A-D(344-347)也产自该菌,对卤虫的LD50分别为1.27、2.11、4.95 和 3.42 μmol/L[141]。黄柄曲霉A. flavipes AIL8代谢产生flavipesins A(348)和B(349),348对金黄色葡萄球菌和枯草芽孢杆菌的MIC分别为8.0 μg/mL和0.25 μg/mL[142]。二倍半萜asperterpenols A(350)和B(351)来自一株内生曲霉Aspergillus sp. 085242,二者对乙酰胆碱酯酶的IC50值分别为2.3和3.0 μmol/L[143]。曲霉菌Aspergillus sp. 16-5c代谢产生二倍半萜asperterpenoid A(352)(图 5),它对分枝杆菌Mycobacteriumtuberculosis蛋白酪氨酸磷酸酶的IC50值为2.2 μmol/L[144]。土曲霉A. terreus GX7-3B代谢产生了噻吩骈萘醌衍生物353[145]。
2.3 其它植物来源的曲霉天然产物吲哚单萜speradine A(354)产自溜曲霉A. tamarii M143,抑制Ca2+-ATP酶的IC50为8 μmol/L、抑制组蛋白去乙酰化酶的IC50为100 μg/mL,抑制藤黄微球菌Mycrococcus luteus的MIC 16.7 μg/mL[146]。6-methoxyspirotryprostatin B(355)、18-oxotryprostatin A(356)、14-hydroxyterezine D(357)、14-norpseurotin A(358)和359(图 5)来自萨氏曲霉A. sydowi PFW1-13,其中化合物355-357对A549细胞的IC50分别为8.29、1.28和7.31 μmol/L,化合物358和359对大肠杆菌、枯草杆菌和溶壁微球菌的MIC分别为3.74、14.97、7.49 μmol/L和10.65、5.33、10.65 μmol/L[147]。
3 海泥、海水等来源的曲霉天然产物 3.1 海泥来源的曲霉天然产物肉色曲霉A. carneusMST-MF156代谢产生aspergillicins A-E(360-364)(图 5),对捻转血矛线虫Haemonchus contortus具有细胞毒性(LD99为25-50 μg/mL)[148]。糖苷类化合物365产自变异曲霉A. varians KMM 4630,其在10.0 μg/mL时对海胆胚胎有毒性[149]。2,3-dimethoxyosoate(366)产自曲霉Aspergillus sp. B-F-2,其对K562细胞的IC50为76.5 μmol/L,在100 μmol/L时可以诱导细胞凋亡、使细胞停滞在S期[150]。烟曲霉A. fumigates 030402d代谢产生11-O-methylpseurotin A(367),对Hof1 缺失的酵母菌Saccharomyces cerevisiae 的抑菌圈直径为9 mm[151]。白曲霉A. candidus RF-5672代谢产生terprenin(368)、3-methoxyterprenin(369)和4"-deoxyterpren(370),其抑制CoA-A 介导的小鼠脾淋巴细胞的IC50依次为1.2、2.0和5.6 ng/mL,抑制LPS介导的小鼠脾淋巴细胞的IC50分别为4.5、8.0、15.6 ng/mL[152]。炭黑曲霉A. carbonarius WZ-4-11代谢产生carbonarones A (371)和B(372)[153]及化合物373-374[154],其中371和372对K562细胞的IC50值分别为56.0和27.8 μg/mL[153],373和374对结核分歧杆菌Mycobacterium tuberculosis H37Rv的MIC依次为43.0和25.1 μmol/L[154]。烟曲霉A. fumigates Fres代谢产生胶霉素375[155]和二酮哌嗪376-378[156]。变色曲霉A. versicolor MST-MF495代谢产生cottoquinazoline A(379)和cotteslosins A(380)和B(381)[157]。氧杂螺内酰胺azaspirofurans A(382)和B(383)来自萨氏曲霉A. sydowi D2-6,382对A549的IC50 10 μmol/L[158]。二酮哌嗪azonazine(384)来自胰岛曲霉A. insulicola 088708a,有抗炎作用,其对NF-κB的IC50为8.37 μmol/L[159]。杂萜asperdemin(385)来自变色曲霉A. versicolor,有溶血作用,EC50 1.15 mmol/L[160]。环肽unguisin E(386)和deoxyapoaranotin(387)分别来自曲霉Aspergillus sp. AF119[161]和变色曲霉A. versicolor KMD 901[162],Aspergillus sp. AF119还代谢产生barceloneic lactones B(388)和C(389)和5'-hydroxychlorflavonin(390)[163]以及terphyl acid(391)、terphyl diacid(392)[164](图 5)。
Protulactones A、B(393、394)[165]及protuboxepins A、B(395、396)、protubonines A、B(397、398)(图 5)来自Aspergillus sp. SF-5044[166],该菌还代谢产生aflaquinolones A-G(399-405)[167]。A. versicolor ZLN-60产生环戊肽versicotides A(406)、B(407)[168]和异戊二烯化的二苯醚衍生物diorcinols B-E(408-411)[169],其中化合物410对Hela和K562细胞的IC50值分别为 31.5 和 48.9 μmol/L、411对Hela细胞的IC50值为36.5 μmol/L[169]。台中曲霉A. taichungensis ZHN-7-07代谢产生prenylterphenyllins A-C(412-414)、4''-dehydro-3-hydroxyterphenyllin(415)及prenylcandidusins A-C(416-418),其中化合物412对HL-60和A549细胞的IC50分别为1.5和8.3 μmol/L、415和417对P388细胞的IC50分别为2.7和1.6 μmol/L[170]。补身烷倍半萜419-423(图 5)来自一株焦曲霉A. ustus,化合物422抑制P388的IC50为8.7 μmol/L[171]。Prenylcyclotryprostatin B(424)、20-hydroxycyclotryprostatin B(425)、9-hydroxyfumitremorgin C(426)、6-hydroxytryprostatin B(427)和spirogliotoxin(428)(图 6)来自烟曲霉A. fumigates YK-7,化合物424和426对U937细胞的IC50分别为25.3 μmol/L和18.2 μmol/L[172]。土曲霉A. terreus A8-4代谢产生7''-hydroxybutyrolactone III(429)和terretriones A-C(430-432)[173];三肽presclerotiotide F(433)来自胰岛曲霉A. insulicola 088708aZA[174];萘烷衍生物decumbenone C(434)来自硫色曲霉A. sulphureus KMM 4640,对人体黑色素瘤SK-MEL-5的细胞毒活性IC50为0.9 μmol/L[175]。二酮哌嗪brevianamides S-V(435-438)来自变色曲霉A. versicolor MF030,化合物435具有选择性地抑制结核分支杆菌Mycobacterium bovis减毒株BCG的活性(MIC为6.25 μg/mL),可能发展为抗结核杆菌先导药物[176]。焦曲霉A. ustus 094102代谢产生倍半萜ustusols A-C(439-441)和ustusolates A-E(442-446)以及香豆素ustusoranes A-F(447-452),其中446和451对HL-60的IC50值分别为9.00 μmol/L和0.13 μmol/L,444对A549细胞的IC50为10.5 μmol/L[177]。外消旋的螺环生物碱effusin A(453)和dihydrocryptoechinulin D(454)(图 6)分离自赭曲霉A. effuses H1-1[178, 179],其中454对P388和HL-60细胞的IC50分别为1.83 μmol/L和4.80 μmol/L、在100 μmol/L时可以选择性的抑制拓扑异构酶I的活性[178].
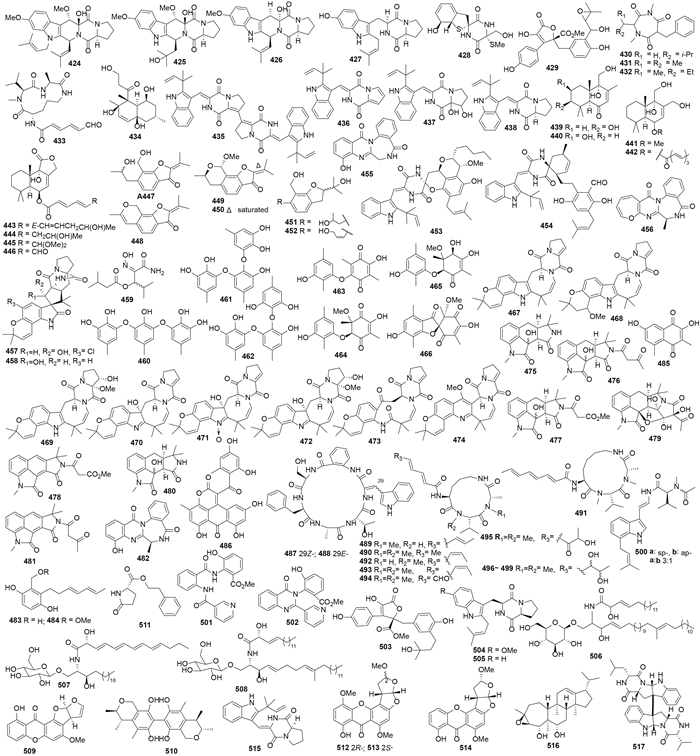
|
| 图6 化合物424–517的结构 Figure 6. Structures of compounds 424–517. |
曲霉A. westerdijkiae DFFSCS013代谢生物碱circumdatins K(455)和L(456)、5-chlorosclerotiamide(457)、10-epi-sclerotiamide(458)和aspergilliamide B(459)(图 6),其中化合物457和458对K562细胞的IC50值分别是44和53 μmol/L[180]。三聚的sydowiols A-C(460-462)产自一株萨氏曲霉A. sydowii MF357,化合物460和462对结核分枝杆菌M. tuberculosis蛋白酪氨酸磷酸酶PtpA的IC50值分别为14 μg/mL和24 μg/mL。此外,化合物462对金黄色葡萄球菌的MIC值为12.5 μg/mL[181]。棘孢曲霉A. aculeatus代谢产生新苯醌aculeatusquinones A-D(463-466),其中464和466对HL-60、K562和A549细胞的IC50值在5.4-76.1 μmol/L之间[182]。杂色曲霉A. versicolor HDN08-60代谢产生吲哚二酮哌嗪versicamides A-H(467-474);其中474对Hela、HCT-116、HL-60和K562细胞的IC50分别为19.4、17.7、8.7 和22.4 μmol/L,474也可抑制多种酪氨酸激酶的活性,10 μmol/L对KDR、RET和EGFR激酶的抑制率为23%到35%,对c-Kit的抑制率为60%[183]。米曲霉A. oryzae代谢产生吲哚生物碱speradines B-E(475-478),475和478对Hela细胞的IC50均为0.20 mmol/L[184];吲哚生物碱speradines F-H(479-481)和circumdatin G(482)分别来自米曲霉A. oryzae[185]和褐黄曲霉A. ochraceus[186]。灰绿曲霉A. glaucus HB1-19代谢产生aspergentisyls A、B(483、484)和aspergiodiquinone(485)(图 6),483和484具有DPPH自由基清除活性,IC50值分别为9.3 μmol/L和17.6 μmol/L[187]。蒽醌类衍生物aspergiolide A(486)产自同一株菌,对A549、HL-60、BEL-7402有细胞毒活性[188]。
菌核曲霉A. sclerotiorum PT06-1在高盐寡营养条件下产生环六肽sclerotides A、B(487、488)[189],在高盐富营养条件下则产生环三肽sclerotiotides A-K(489-499)[190]和indole-3-ethenamide(500)[191];其中化合物487和488均显示中等强度的抗白色念珠菌活性、MIC值分别为7.0 μmol/L和3.5 μmol/L,化合物488对HL-60细胞和铜绿假单胞菌有抑制作用、IC50和MIC值分别为56.1和35.3 μmol/L;化合物489、490、494和497对白色念珠菌有选择性抑制作用,MIC分别为7.5、3.8、30.0和6.7 μmol/L,化合物500对A549和HL-60细胞的IC50分别为3.0和27.0 μmol/L。土曲霉A. terreus PT06-2在盐胁迫条件下代谢产生terremides A、B(501-502)和terrelactone A(503),其中501对金黄色葡萄球菌的MIC为63.9 μmol/L、502对产气肠杆菌的MIC为33.5 μmol/L[192]。烟曲霉A. fumigates BM939代谢产生对映异构的tryprostatins A(504)和B(505)(图 6),浓度分别为50 μg/mL和12.5 μg/mL时,两者均能将tsFT-210的细胞周期阻滞于G2/M期[193]。
3.2 海水来源的曲霉天然产物Asperiamide A(506)产自曲霉Asperillus sp. MF-34[194],asperiamides B(507)和C(508)则来自黑曲霉A. niger MF-16[195]。曲霉Aspergillussp. MF-93代谢产生asperxanthone(509)和asperbiphenyl(510)(图 6),均可阻断烟草花叶病毒TMV的复制,0.2 mg/mL浓度下的抑制率分别为62.9%和35.5%[196]。杂色曲霉A. versicolor ZBY-3的新霉素耐药菌株u2n2h3-3代谢产生5-oxo-L-prolinate(511),其对Hela细胞的IC50值为49.0 μg/mL[197]。柄曲菌素类oxisterigmatocystins A-C(512-514)[198]和二酮哌嗪brevianamide W(515)[199]来自变色曲霉A. versicolor CXCTD-06-6a,其中515在13.9 μmol/L时对DPPH的清除率为55%。
3.3 未知来源的曲霉天然产物二倍半萜aspergilloxide(516)和二聚二酮哌嗪517(图 6)分别来自曲霉Aspergillus sp. CNM-713[200]和黑曲霉A. niger[201]。
4 结论和展望从1992年Shinggu等首次报道海洋曲霉来源的新天然产物fumiquinazolines A-C[2](Numata et al. 1992)到2014年8月,已发现海洋曲霉来源的新天然产物512个。海洋曲霉天然产物的结构类型多样,包括聚酮、生物碱、萜类、甾体、脂肪酸、肽类及其卤代物和糖苷等;且36%的化合物表现出抗癌(肿瘤细胞毒)、抑菌、抗氧化(自由基清除)和抗寄生虫等生物活性,是发现活性新天然产物和药物先导物的重要资源。
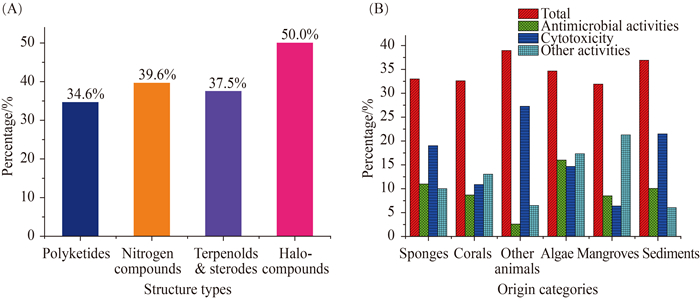
(1) 从海洋曲霉菌的样品来源看,产生新化合物最多的曲霉菌来源或栖息地依次是海泥(149个)、海绵(100个)、其它动物(77个)和海藻(75个),分别占29%、20%、15%和15%(图 7-A)。

|
| 图7 海洋曲霉天然产物的来源(A)与结构分类(B) Figure 7. Origin categories (A) and the main structure types (B) of marine-derived Aspergillus fungal NPs. |
(2) 从化合物类型看,化合物最多的类型依次是含氮(265个)、聚酮(153个)和萜甾(96个),分别占化合物总数的52%、30%和19%(图 7-B)。进一步分析其来源,聚酮类化合物产生菌的主要栖息地是海泥和海绵,分别为26%和22%;萜甾化合物产生菌的主要栖息地是海绵和海藻,分别为38%和25%;含氮化合物产生菌的主要栖息地是海泥,为35% (图 8-A)。进一步结合来源分析,珊瑚、海藻和海泥来源的曲霉主要代谢产生含氮化合物,分别为48%、51%和63%;红树林来源的曲霉主要代谢产生聚酮化合物,约为64%;而海绵来源的曲霉天然产物的结构类型相对均衡,聚酮、含氮化合物及萜甾分别为33%、27%和36% (图 8-B)。
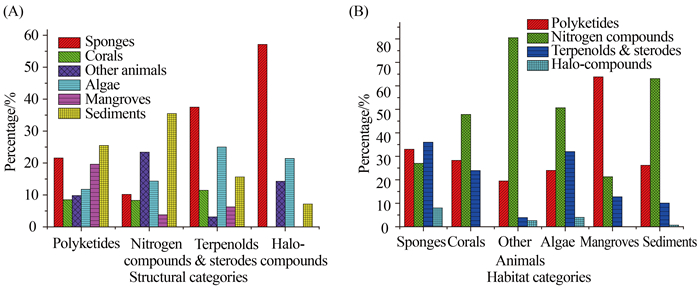
|
| 图8 主要结构类型化合物产生菌的栖息地(A)与不同样品来源的曲霉天然产物的结构类群(B) Figure 8. Habitat categories on the main structures of producing strains (A) and structural categories (B) of NPs from marine-derived Aspergillus fungi. |
(3) 约36%的海洋曲霉天然产物(184个)表现出细胞毒、抗菌、自由基清除和抗寄生虫等多种生物活性(图 9-A和表 1),而肿瘤细胞毒(94个)和抗菌(51个)是其主要的活性,分别占活性化合物总数的51%和28%。含氮物、聚酮和萜甾是活性化合物的三大类型,分别占活性化合物总数的57%、29%和20% (图 9-B);卤代物、含氮物、萜甾、聚酮和肽类出现活性化合物的比例分别为各类化合物总数的50%、40%、41%、38%和35% (图 10-A);而海泥和海绵来源的曲霉最容易产生肿瘤细胞毒活性的化合物,其概率分别为21%和19% (图 10-B)。
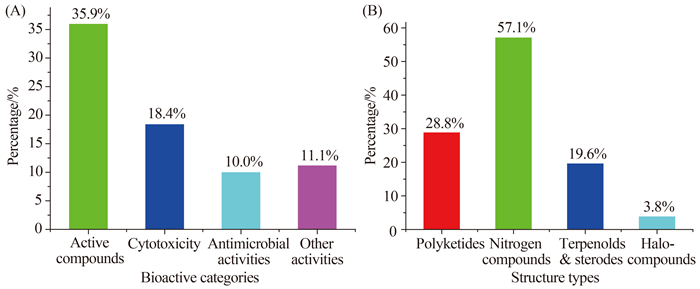
|
| 图9 海洋曲霉天然产物的活性分类(A)与活性化合物中各结构类型的比例(B) Figure 9. Bioactive categories of NPs (A) and ratios of the bioactive NPs from structural types (B) of the marine-derived Aspergillus fungal origins. |
|
| 图10 不同结构类型化合物的活性率(A)与产生菌来源化合物的活性率(B) Figure 10. Ratios of active NPs from the structural types (A) and the sources of the Aspergillus fungi (B). |
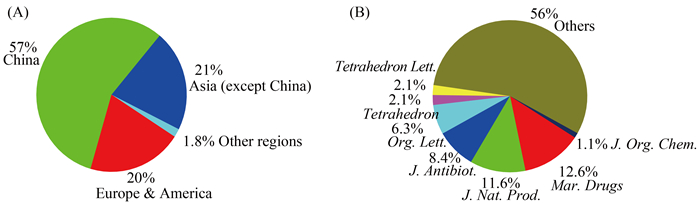
(4) 我国、其它亚洲国家和欧美国家在海洋天然产物的发展上占据了重要地位,分别贡献287、108和103个新化合物,尤其是我国海洋天然产物化学家贡献了57%的海洋曲霉来源的新天然产物(图 11-A)。本文共引用了95篇(占引用文献总数的53%)我国海洋天然产物化学家发表的文章,是主要的贡献者,标志我国海洋天然产物的研究具有一定的国际影响力。但其发表在有机化学类或天然产物化学类的主流杂志如Org. Lett.、J. Org. Chem.、Tetrahedron和J. Nat. Prod.分别仅有6、1、2和11篇,仅占其文章的6.3%、1.1%、2.1%和11.6%,且未见其在J. Am. Chem. Soc.和Angew. Chem.,Int. Ed.等化学综合类高水平杂志上发表文章(图 11-B),也未见有药进入临床研究。由此可见,我国曲霉菌海洋天然产物的研究,应该注重质和国家需求,而非单纯的量或发表文章。
|
| 图11 海洋曲霉天然产物发现者国别(A)和中国学者发表论文的期刊分类(B) Figure 11. Country categories of the discoverers (A) and publishing Journal categories of the Chinese scholars (B) on the marine-derived fungal NPs |
综上,海洋曲霉可以产生结构新颖,活性多样的天然产物,具有极大的开发潜能,但是到目前为止仅有1个来源于海洋曲霉的药物,如何最大限度利用海洋曲霉这类珍贵的微生物资源来开发药物,是值得科研工作者思考和解决的问题。一方面要通过培养基改造、表观遗传修饰和共培养等手段,继续发现新的活性天然产物;另一方面也是最重要的,是如何开展对现有活性天然产物的成药性研究,而化合物的量依然是制约成药性研究的瓶颈因素。发酵工程、代谢工程是开展微生物活性产物规模化制备、获取足量产物的有效方法,而合成生物学为代谢工程有效而系统的分子生物学工具。由于真菌的基因组较大、酶系复杂,制约着其合成生物学的研究。近年来,曲霉次级代谢产物合成酶系及其相关基因结构、功能的研究取得了很大的进展,多个曲霉的基因组序列被公开。一些曲霉属真菌次级代谢产物的生合成机理被阐明,为曲霉属真菌活性天然产物的规模化制备和工业化生产提供了技术和理论基础。如张立新课题组发现真菌毒素verruculogen中的过氧桥键是由一个依赖α-酮戊二酸的单核非血红素酶FtmOx1催化合成的[202]。降血脂的一线药物洛伐他汀(lovastatin)最初是从曲霉中发现,目前控制洛伐他汀的生物合成酶被发现,并通过曲霉发酵实现工业化生产[203, 204];洛伐他汀的衍生物辛伐他汀(simvastatin)也是一种有效的调血脂药物,经典的合成方法是从洛伐他汀出发,经过水解(得到关键中间体monacolin J)、保护、酰化和脱保护等多步反应得到。Yi Tang等在解析洛伐他汀生物合成中酰化酶的基础上,利用全细胞培养,在大肠杆菌(Escherichia coli)过表达酰基转移酶lovD,实现了辛伐他汀的高效合成(转化monacolin J为simvastatin的转化率达到99%以上,且产量达到克级:4?6 g/L)[205, 206]。这些研究成果为海洋曲霉属真菌药物的开发提供了重要的参考。
| Compounds | Producing Strains | Environments source | Bioactivity | References |
| a /: no bioactivity was reported. | ||||
| 1, 2 | A. cf. ochraceus 941026 | Jaspis of Coriacea, Indian-Pacific Ocean | / a | 8 |
| 3 | A. nigere | Hyrtios sp., Florida, America | Cytotoxicity | 9 |
| 4 | A. niger 94–1212 | Hyrtios proteus, Florida, America | / | 10 |
| 5–14 | A. versicolor(Vuill)Triab | Xestospongia exigua, Bali Island, Indonesia | / | 11–12 |
| 15–25 | A. ostianus TUF 01F313 | Unidentified sponge, Pohnpei, Micronesia | 15–17: Antibacterial activity, 22–24: Cytotoxicity | 13–16 |
| 26–28 | A. ostianus IBT 12704 | Unidentified sponge, Pohnpei, Micronesia | / | 17 |
| 29–32 | Aspergillus sp. CNK-371 | Unidentified sponge, Hawaii State | 29–31: Cytotoxicity | 18 |
| 33, 34 | A. aculeatus CRI323-04 | Xestospongia testudinaria, Phi Phi Island, Thailand | 33: α-glucosidase inhibition, Antibacterial activity | 19–20 |
| 35–37 | A. aculeatus CRI322-03 | Unidentified sponge, Phi Phi Island, Thailand | / | 21 |
| 38, 39 | A. insuetus | Petrosia ficiformis, Santa Ana Alhambra Nestorius, Spain | Inhibitor of the mammalian mitochondrial respiratory chain | 22 |
| 40 | A. sclerotiorum Huber SP080903f04 | Mycale sp. Okinawa Island, Japan | / | 23 |
| 41–54 | A. ustus 8009 | Suberites domuncula, The Adriatic Sea | 44, 45: Cytotoxicity | 24–25 |
| 55 | A. versicolor | Petrosia sp., Jeju Island, Korea | Cytotoxicity | 26 |
| 56, 57 | Aspergillus sp. fs14 | Unidentified sponge, Okinawa Island, Japan | / | 27 |
| 58–61 | A. insuetus OY-207 | Psammocinia sp., Israel | 58: Antibacterial activity 60: Cytotoxicity | 28 |
| 62 | A. versicolor PF10M | Petrosia sp., Jeju Island, Korea | Cytotoxicity | 29 |
| 63–69 | Aspergillus sp. | Xestospongia testudinaria, South China Sea, China | 63–66: Antibacterial activity 67, 69: Cytotoxicity | 30–31 |
| 70–74 | A. unguis CRI282-03 | Unidentified sponge CRI282, Thailand | 70–72: Aromatase inhibition 70, 71: XXO scavenging activity | 32 |
| 75 | Eurotium cristatum KUFC 7356 | Mycale sp. State Beach, Thailand | / | 33 |
| 76–89 | Aspergillus sp. | Tethya aurantium, Mediterranean, Italy | 81: Cytotoxicity 88, 89: Antibacterial activity | 34–36 |
| 90–92 | A. versicolor MF359 | Hymeniacidon perleve, Bohai, China | 92: Antibacterial activity | 37 |
| 93–99 | A. niger | Axinella damicornis, Elba, Italy | 93–95: Cytotoxicity | 38 |
| 100 | Aspergillus niger FT–0554 | Unidentified sponge, Palau | Inhibit Ascarissuum | 39 |
| 101, 102 | A. terreus HKI0499 | Sinularia kavarattiensis, Amanda Pam, India | / | 40 |
| 103–107 | Aspergillus sp. | Dichotella gemmacea, Weizhou Island, China | 103: Antibacterial activity 106: Cytotoxicity, fouling resistance | 41–42 |
| 108–112 | A. sydowii PSU-F154 | Annella sp., Surat Thani, Thailand | / | 43 |
| 113–121 | A. versicolor LCJ-5-4 | Cladiella sp., South China Sea, China | 116: DPPH radical scavenging activity 121: Antibacterial activity | 44–45 |
| 124, 125 | A. fumigates | Zoanthus sp., Kagoshima, Japan | / | 49 |
| 126–128 | A. fumigatus KMM 4631 | Sinularia sp., Ostrov Kunashir Island | 126: Planta growth Promotion | 50–51 |
| 129 | A. sydowii SCSIO 00305 | Verrucella umbraculum, Sanya, China | / | 52 |
| 130, 131 | Aspergillus sp. | Zoanthus sp., Kagoshima, Japan | Cytotoxicity | 53–54 |
| 132–134 | A. terreus SCSGAF0162 | Echinogorgia aurantiaca, Sanya, China | 132: Cytotoxicity, Antivirus | 55 |
| 135–138 | Aspergillus sp. XS-20090066 | Dichotella gemmacea, Xisha Islands, South China Sea | / | 56 |
| 139–141 | A. elegansZJ-2008010 | Sarcophyton sp., Weizhou Island, China | 139: Antibacterial activity | 57 |
| 142 | Aspergillus sp. SCSGAF 0076 | Melitodes squamata, Sanya, China | / | 58 |
| 143, 144 | Eurotium rubrum SH-823 | Sarcophyton sp., South China Sea, China | Anti-α-glycosides | 59 |
| 145, 146 | A. versicolor | Dichotella gemmacea, South China Sea, China | Antibacterial activity, Anti-brine shrimp activity | 60 |
| 147, 148 | A. flavipes | Anthopleura xanthogrammica, Qingdao, China | Cytotoxicity | 61 |
| 149–156 | A. fumigatus OUPS-T106B-5 | Pseudolabrus japonicus, Tanabe Bay, Japan | Cytotoxicity | 2, 64–65 |
| 157–159 | A. fumigatus OUPS-T106B-5 | Pseudolabrus japonicus, Tanabe Bay, Japan | 158, 159: Cytotoxicity | 66 |
| 160–161 | A. terreus OUCMDZ-1925 | Chelon haematocheilus, Yellow River estuary, China | DPPH radical scavenging; Cytotoxicity; Anti-virus | 67 |
| 162–167 | Aspergillus sp. MF275 | Mytilus edulis, Toyama Bay, Japan | 162: Ubiquitin-activating enzyme (E1) inhibitor | 68–69 |
| 168–190 | Aspergillus sp. MF297-2 | Mytilus edulis, Japan | 170, 175: Cytotoxicity | 70–75 |
| 191 | A. fumigatus OUPS-N138 | Toxopneustes pileolus, Japan | / | 76 |
| 192–197 | A. versicolor OUPS-N136 | Anthocidaris crassispana, Tanabe Bay, Wakayama, Japan | 193–195: Cytotoxicity | 77 |
| 198–200 | Aspergillus sp. HDf2 | Anthocidaris crassispina, Hainan, China | / | 78 |
| 201–203 | A. clavatus C2WU | Xenograpsus testudinatus, Taiwan, China | 201–202: Cytotoxicity | 79–80 |
| 204–210 | A. fumigatus | S. japonicus, Lingshan Island, Qingdao, China | 207–210: Cytotoxicity | 81 |
| 211–212 | A. fumigatus WFZ-25 | S. japonicus, Jiaozhou Bay, China | / | 82 |
| 213–220 | A. niger F97S11 | Aplidium sp., Fiji | / | 83 |
| 221 | Aspergillus sp. MF297 | Mytilus edulis, Toyama Bay, Japan | Cytotoxicity | 84 |
| 222–224 | A. flavus OUCMDZ-2205 | Penaeus vannamei, Lianyungang sea area, China | 222: Antibacterial activity 222–223: Cytotoxicity | 85 |
| 225 | A. flavipes Z-4 | Ligia oceanica, Oceania | / | 86 |
| 226–227 | Aspergillus sp. 05F16 | Unidentified marine alga, Indonesia | / | 87 |
| 228 | A. insulicola | Unidentified marine alga, Bahamas | / | 88 |
| 229–232 | A. versicolor CNC 327 | Penicillus capitatus, Caribbean | 229: Cytotoxicity | 89 |
| 233 | Aspergillu sp. | Sargassum sp., Philippines | 233: Antibacterial activity | 90 |
| 234 | A. parasiticus MFA153 | Carpopeltis cornea, Korea | / | 91 |
| 235 | A. terreus MFA 460 | Halymenia acuminata, Korea | UV-A absorbing activity | 92 |
| 236 | Aspergillus sp. MFA 212 | Lomentaria catenata, Ulsan, Korea | UV-A absorbing activity; DPPH radical scavenging | 93 |
| 237–238 | A. sydowii | Acanthophora spicifera, Bay of Bengal India | / | 94 |
| 239 | Aspergillus sp. MFB024 | Sargassum horneri, Korea | Antibacterial activity | 95 |
| 240–248 | A. niger EN-13 | Colpomenia sinuosa, Qingdao, China | 242: Antibacterial activity; DPPH radical scavenging 243: Antibacterial activity | 96–100 |
| 249–251 | A. flavus c-f-3 | Enteromorpha tubulosa, Putian, China | 251: Cytotoxicity | 101–102 |
| 252–255 | A. ochraceus EN-31 | Sargassum kjellmanianum, Daliancoastline, China | 252: DPPH radical scavenging | 103–104 |
| 256–259 | A. oryzaer cf-2 | Heterosiphonia japonica, Yantai, China | 258: Antibacterial activity | 105–106 |
| 260–261 | Aspergillus sp. SpD081030G1f1 | Sargassum sp., Ishigaki Island, Japan | DPPH radical scavenging | 107 |
| 262–263 | A. flavus | Codium fragile,Yeosu, Korea | Antibacterial activity | 108 |
| 264–265 | A. flavus cf-5 | Corallina officinalis, Yantai, China | / | 109 |
| 266–277 | A. carneus KMM4638 | Laminaria sachalinensis, Kunachir Island | / | 110–111 |
| 278–282 | A. wentii EN-48 | Sargassum sp., Unknown place | 278–282: Cytotoxicity 282: DPPH radical scavenging | 112–113 |
| 283 | A. versicolor | Halimeda opuntia, Egyptian Red Sea (5–8 m) | Antibacterial activity | 114 |
| 284–291 | A. ustus cf42 | Codium fragile, Zhoushan Island, China | 287: Antibacterial activity | 115 |
| 292–293 | A. versicolorpt20 | Sargassum thunbergii, Pingtan Island, China | / | 116 |
| 294–295 | A. versicolor dl29 | Codium fragile, Dalian, China | 294: Anti-brine shrimp activity, Antibacterial activity 295: Inhibition of H. akashiwo | 117–118 |
| 296 | A. versicolor EN-7 | Sargassum thunbergii, Qingdao, China | / | 119 |
| 297 | E. herbariorum HT-2 | Enteromorpha prolifera, Qingdao, China | Cytotoxicity, Antibacterial activity | 120 |
| 298 | A. ochraceus Jcma1F17 | Coelarthrum sp., Paracel Islands, China | Cytotoxicity, Anti- H3N2 and EV71 activity | 121 |
| 299 | Aspergillus sp. BM-05 and BM-05ML | Sargassum sp., Helgoland, North Sea, Germany | Cytotoxicity | 122 |
| 300 | A. pseudodeflectus | Sargassum fusiform, Miura Peninsula, Japan | Cytotoxicity | 123 |
| 301 | Aspergillus sp. CNC139 A. ustus NSC-F038 | Halimeda copiosa, Philippines | Cytotoxicity | 3, 4 |
| 305 | A. niger LL-LV3020 | Mangrove wood, Hong Kong, China | / | 131 |
| 306–310 | A. favipes | Mangrove Plant Acanthus ilicifolius, Dongzhai Gang, China | 132 | |
| 311–314 | Eurotium rubrum QEN-0407-G2 | Marine mangrove plant Hibiscus tiliaceus, Hainan Island, China | 311: DPPH radical scavenging | 133 |
| 315–321 | A. tubingensis GX1-5E | Radix of Pongamia pinnata, South China Sea, Guangxi | / | 134–135 |
| 322–331 | A. niger MA132 | Mangrove, Hainan, China | 323, 326: Cytotoxicity | 136 |
| 332–333 | Aspergillus sp. 085241B | Mangrove, Shankou, Guangxi, China | / | 137 |
| 334 | A. flavus 092008 | Mangrove plant, Hainan, China | Cytotoxicity, Antibacterial activity | 138 |
| 335–336 | Aspergillus sp. | Bruguiera gymnorrhiza, South China Sea, China | / | 139 |
| 337–347 | A. nidulans MA-143 | Rhizophora stylosa, Unknown place | 338–339, 343, 344–347: Anti-brine shrimp activity | 140–141 |
| 348–349 | A. flavipes AIL8 | Acanthus ilicifolius, Daya Bay, Shenzhen, China | Antibacterial activity | 142 |
| 350–351 | Aspergillus sp. 085242 | Mangrove plant, Guangxi, China | Acetylcholinesterase inhibition | 143 |
| 352 | Aspergillus sp. 16-5c | Mangrove plant, South China Sea, China | mPTPB inhibition | 144 |
| 353 | A. terreus GX7-3B | Bruguiera gymnoihiza, Guangxi, China | / | 145 |
| 354 | A. tamarii M143 | Driftwood, Okinawa Island, Iapan | Ca2+-ATPase inhibition, Antibacterial activity | 146 |
| 355–359 | A. sydowi PFW1-13 | Driftwood, Baishamen,Hainan, China | Cytotoxicity, Antibacterial activity | 147 |
| 360–364 | A. carneus MST-MF156 | Sediment, Jordan River Bridge, Tasmania, Australia | Antiparasitic activity | 148 |
| 365 | A. varians KMM 4630 | Sediment, Sakhalin Island | Cytotoxicity | 149 |
| 366 | Aspergillus sp. B-F-2 | Sediment, Behai Bay, China | Cytotoxicity | 150 |
| 367 | A. fumigates 030402d | Sediment (>30 m), Vanuatu | Antimicrobial activity | 151 |
| 368–370 | A. candidus RF-5672 | Sediment, Shodo Island, Kagawa Prefecture, Japan | Cytotoxicity | 152 |
| 371–374 | A. carbonarius WZ-4-11 | Sediment, Weizhou Island, China | 371–372: Cytotoxicity; 373–374: Antimicrobial activity | 153–154 |
| 375–378 | A. fumigates Fres | Sediment, Jiaozhou Bay, Qingdao, China | / | 155–156 |
| 379–381 | A. versicolor MST-MF495 | Beach sand sample, Cottesloe, Western Australia | / | 157 |
| 382, 383 | A. sydowi D2–6 | Sediment, Jiaozhou Bay, Qingdao, China | 382: Cytotoxicity | 158 |
| 384 | A. insulicola 088708a | Sediment, Hawaii | Anti-inflammation | 159 |
| 385 | A. versicolor | Sediment, Sakhalin Bay, Russian | Antihemolysis | 160 |
| 386 | Aspergillus sp. AF119 | Sediment, Xiamen beach, China | / | 161 |
| 387 | A. Versicolor KMD 901 | Sediment, East Sea, Korea | / | 162 |
| 388–392 | Aspergillus sp. AF119 | Sediment, Xiamen beach, China | / | 163–164 |
| 393–405 | Aspergillus sp. SF-5044 | Sediment, Dadaepo Beach, Busan, Korea | / | 165–167 |
| 406–411 | A. versicolor ZLN-60 | Sediment, Yellow Sea | 410, 411: Cytotoxicity | 168–169 |
| 412–418 | A. taichungensis ZHN-7-07 | Root soil of the mangrove plant Acrostichum aureum | 412, 415, 417: Cytotoxicity | 170 |
| 419–423 | A. ustus | Rhizosphere soil of the mangrove Acrostichum aureurm, Guangxi, China | 422: Cytotoxicity | 171 |
| 424–428 | A. fumigates YK-7 | Sediment, Yingkou, China | 424, 426: Cytotoxicity | 172 |
| 429–432 | A. terreus A8-4 | Mangrove-associated marine sediments, Guangxi, China | / | 173 |
| 433 | A. insulicola 088708aZA | Sediment, Hawaii | / | 174 |
| 434 | A. sulphureus KMM 4640 | Sediment, Unknown place | Cytotoxicity | 175 |
| 435–438 | A. versicolor MF030 | Sediment, Bohai Sea, China | 435: Antitubercular activity | 176 |
| 439–452 | A. ustus 094102 | Rhizosphere soil of the mangrove plant Bruguiera gymnorrhiza, Wenchang, Hainan, China | 444, 446, 451: Cytotoxicity | 177 |
| 453, 454 | A. effuses H1-1 | Mangrove rhizosphere soil, Fujian, China | 454: Cytotoxicity | 178–179 |
| 455–459 | A. westerdijkiae DFFSCS013 | Sediment (–2918 m), South China Sea | 457, 458: Cytotoxicity | 180 |
| 460–462 | A. sydowii MF357 | Sediment, Bohai Sea, China | 460, 462: Antimicrobial activity | 181 |
| 463–466 | A. aculeatus | Sediment, Langqi Island, Fujian, China | 464, 466: Cytotoxicity | 182 |
| 467–474 | A. versicolor HDN08-60 | Sediment, South China Sea | 474: Cytotoxicity; Inhibition of PTKs | 183 |
| 475–478 | A. oryzae | Sediment, Langqi Island, Fujian, China | 475, 478: Cytotoxicity | 184 |
| 479–481 | A. oryzae | Sediment, Langqi Island, Fujian, China | / | 185 |
| 482 | A. ochraceus | Sediment, Sea of Japan | / | 186 |
| 483–485 | A. glaucus HB1-19 | Mangrove rhizosphere soil, Fujian, China | 483, 484: DPPH-radical scavenging | 187 |
| 486 | A. glaucus HB1-19 | Mangrove rhizosphere soil, Fujian, China | Cytotoxicity | 188 |
| 487–500 | A. sclerotiorum PT06-1 | Putian Sea Salt Field, Fujian, China | 487–490, 494, 497: Antimicrobial activity 488, 500: Cytotoxicity | 189–191 |
| 501–503 | A. terreus PT06-2 | Putian Sea Salt Field, Fujian, China | 501, 502: Antimicrobial activity | 192 |
| 504, 505 | A. fumigates BM939 | Sediment (–760 m), Oi River, Japan | Cytotoxicity | 193 |
| 506 | Asperillus sp. MF-34 | Sea water, Mei-Zhou Gulf, Fujian, China | / | 194 |
| 507, 508 | A. niger MF-16 | Sea water, Quanzhou Gulf, Fujian, China | / | 195 |
| 509, 510 | Aspergillus sp. MF-93 | Sea water, Quanzhou Gulf, Fujian, China | Antivirus | 196 |
| 511 | A. versicolor ZBY-3 | Sea water (–800 m), Southeast Pacific | Antimicrobial activity; Cytotoxicity | 197 |
| 512–515 | A. versicolor CXCTD-06-6a | Sea water (–800 m), Pacific Ocean | 515: DPPH-radical scavenging | 198–199 |
| 516 | Aspergillus sp. CNM-713 | Unknown source | / | 200 |
| 517 | A. niger | Unknown source | / | 201 |
致谢
本课题组的王聪博士和马颖娜硕士在本文的写作及文献查阅过程中付出了大量艰辛的劳动,在此对其贡献表示感谢。
| [1] | Zhao CY, Zhu TH, Zhu WM. New marine natural products of microbial origin from 2010 to 2013. Chinese Journal of Organic Chemistry, 2013, 33(06): 1195-1234 (in Chinese)赵成英, 朱统汉, 朱伟明. 2010-2013 之海洋微生物新天然产物. 有机化学, 2013, 33(06): 1195-1234. |
| [2] | Numata A, Takahashi C, Matsushita T, Miyamoto T, Kawai K, Usami Y, Matsumura E, Inoue M, Ohishi H, Shingu T. Fumiquinazolines, novel metabolites of a fungus isolated from a salt fish. Tetrahedron Letters, 1992, 33(12): 1621-1624. |
| [3] | Fenical W, Jensen PR, Cheng XC. Halimide, a cytotoxic marine natural product, derivatives thereof and therapeutic use in inhibition of proliferation. PCT Int. Appl. WO 9948889. 19990930. |
| [4] | Fenical W, Jensen P, Cheng X. Halimide, a cytotoxic marine natural product and derivatives thereof. US: US 6069146. 20000530. |
| [5] | Yamazaki Y, Tanaka K, Nicholson B, Deyanat G, Potts B, Yoshida T, Oda A, Kitagawa T, Orikasa S, Kiso Y. Synthesis and structure- activity relationship study of antimicrotubule agents phenylahistin derivatives with a didehydropiperazine-2, 5-dione structure. Journal of Medicinal Chemistry, 2012, 55(3): 1056-1071. |
| [6] | Beondspring, 2015. http://www.beyondspringpharma.com/clinical-trials/phase-3/ |
| [7] | Gerwick WH, Moore BS. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Journal of Biological Chemistry, 2012, 19(1): 85-98. |
| [8] | Abrell LM, Borgeson B, Crews P. Chloro polyketides from the cultured fungus (Aspergillus) separated from a marine sponge. Tetrahedron Letters, 1996, 37(14): 2331-2334. |
| [9] | Varoglu M, Corbett TH, Valeriote FA, Crews P. Asperazine, a selective cytotoxic alkaloid from a sponge-derived culture of Aspergillus niger. Journal of Organic Chemistry, 1997, 62(21): 7078-7079. |
| [10] | Varoglu M, Crews P. Biosynthetically diverse compounds from a saltwater culture of sponge-derived Aspergillus niger. Journal of Natural Products, 2000, 63(1): 41-43. |
| [11] | Lin WH, Fu HZ, Li J, Proksch P. Novel chromone derivatives from marine fungus Aspergillus versicolor isolated from the sponge Xestospongia exigua. Chinese Chemical Letters, 2001, 12(3): 235-238. |
| [12] | Lin WH, Li J, Fu HZ, Proksch P. Four novel hydropyranoindeno-derivatives from marine fungus Aspergillus versicolor. Chinese Chemical Letters, 2001, 12(5): 435-438. |
| [13] | Namikoshi M, Negishi R, Nagai H, Dmitrenok A, Kobayashi H. Three new chlorine containing antibiotics from a marine-derived fungus Aspergillus ostianus collected in Pohnpei. The Journal of Antibiotics(Tokyo), 2003, 56(9): 755-761. |
| [14] | Kito K, Ookura R, Yoshida S, Namikoshi M, Ooi T, Kusumi T. Pentaketides relating to aspinonene and dihydroaspyrone from a marine-derived fungus Aspergillus ostianus. Journal of Natural Products, 2007, 70(12): 2022-2025. |
| [15] | Kito K, Ookura R, Yoshida S, Namikoshi M, Ooi T, Kusumi T. New cytotoxic 14-membered macrolides from marine-derived fungus Aspergillus ostianus. Organic Letters, 2008, 10(2): 225-228. |
| [16] | Kito K, Ookura R, Kusumi T, Namikoshi M, Ooi T. X-ray structures of two stephacidins, heptacyclic alkaloids from the marine-derives fungus Aspergillus astianus. Heterocycles, 2009, 78(8): 2101-2106. |
| [17] | Rahbæ k L, Breinholt J. Circumdatins D, E, and F: further fungal benzodiazepine analogues from Aspergillus ochraceus. Journal of Natural Products, 1999, 62(6): 904-905. |
| [18] | Cueto M, MacMillan JB, Jensen PR, Fenical W. Tropolactones A- D, four meroterpenoids from a marine-derived fungus of the genus Aspergillus. Phytochemistry, 2006, 67(16): 1826-1831. |
| [19] | Ingavat N, Dobereiner J, Wiyakrutta S, Mahidol C, Ruchirawat S, Kittakoop P. Aspergillusol A, an α-glucosidase inhibitor from the marine-derived fungus Aspergillus aculeatus. Journal of Natural Products, 2009, 72(11): 2049-2052. |
| [20] | Ingavat N, Mahidol C, Ruchirawat S, Kittakoop P. Asperaculin A, a sesquiterpenoid from a marine-derived fungus Aspergillus aculeatus. Journal of Natural Products, 2011, 74(7): 1650-1652. |
| [21] | Antia BS, Aree T, Kasettrathat C, Wiyakrutta S, Ekpa OD, Ekpe UJ, Mahidol C, Ruchirawat S, Kittakoop P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry, 2011, 72(8): 816-820. |
| [22] | Ló pez-Gresa MP, Cabedo N, Gonzalez-Mas MC, Ciavatta ML, Avila C, Primo J. Terretonins E and F, inhibitors of the mitochondrial respiratory chain from the marine-derived fungus Aspergillus insuetus. Journal of Natural Products, 2009, 72(7): 1348-1351. |
| [23] | Motohashi K, Inaba S, Takagi M, Shin-ya K. JBIR-15, a new aspochracin derivative isolated from a sponge-derived fungus, Aspergillus sclerotiorum Huber Sp080903f04. Biosci Biotechnol Biochem, 2009, 73(8): 1898-1900. |
| [24] | Liu H, Edrada-Ebel R, Ebel R, Wang Y, Schulz B, Draeger S, Muller WE, Wray V, Lin W, Proksch P. Drimane sesquiterpenoids from the fungus Aspergillus ustus isolated from the marine sponge Suberites domuncula. Journal of Natural Products, 2009, 72(9): 1585-1588. |
| [25] | Liu H, RuAngelie RE, Yao BS, Siegfried WEG. Ophiobolin sesterterpenoids and pyrrolidine alkaloids from the sponge-derived fungus Aspergillus ustus. Helvetica Chimica Acta, 2011, 94(4): 623-631. |
| [26] | Lee Y, Dang HT, Hong J, Lee CO, Bae K, Kim D, Jung JH. A cytotoxic lipopeptide from the sponge-derived fungus Aspergillus versicolor. Bulletin of the Korean Chemical Society, 2010, 31(1): 205-208. |
| [27] | Takagi M, Motohashi K, Shin-ya K. Isolation of 2 new metabolites, JBIR-74 and JBIR-75, from the sponge-derived Aspergillus sp. fS14. The Journal of Antibiotics (Tokyo), 2010, 63(7): 393-395. |
| [28] | Cohen E, Koch L, Thu KM, Rahamim Y, Aluma Y, Ilan M, Yarden O, Carmeli S. Novel terpenoids of the fungus Aspergillus insuetus isolated from the Mediterranean sponge Psammocinia sp. collected along the coast of Israel. Bioorganic and Medicinal Chemistry, 2011, 19(22): 6587-6593. |
| [29] | Lee Y, Dang HT, Li J, Zhang P, Hong J, Lee CO, Jung JH. A cytotoxic fellutamide from the sponge-derived fungus Aspergillus versicolor. Bulletin of the Korean Chemical Society, 2011, 32(10): 3817-3820. |
| [30] | Li D, Xu Y, Shao CL, Yang RY, Zheng CJ, Chen YY, Fu XM, Qian PY, She ZG, de Voogd NJ, Wang CY. Antibacterial bisabolane-type sesquiterpenoids from the sponge-derived fungus Aspergillus sp. Marine Drugs, 2012, 10(1): 234-241. |
| [31] | Sun L, Shao C, Chen J, Guo Z, Fu X, Chen M, Chen Y, Li R, de Voogd NJ, She Z. New bisabolane sesquiterpenoids from a marine-derived fungus Aspergillu sp. isolated from the sponge Xestospongia testudinaria. Bioorganic and Medicinal Chemistry Letters, 2012, 22(3): 1326-1329. |
| [32] | Sureram S, Wiyakrutta S, Ngamrojanavanich N, Mahidol C, Ruchirawat S, Kittakoop P. Depsidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282-03. Planta Medica, 2012, 78(06): 582-588. |
| [33] | Gomes NM, Dethoup T, Singburaudom N, Gales L, Silva A, Kijjoa A. Eurocristatine, a new diketopiperazine dimer from the marine sponge-associated fungus Eurotium cristatum. Phytochemistry Letters, 2012, 5(4): 717-720. |
| [34] | Zhou Y, Má ndi A, Debbab A, Wray V, Schulz B, Mü ller WEG, Lin W, Proksch P, Kurtá n T, Aly AH. New austalides from the sponge-associated fungus Aspergillus sp. European Journal of Organic Chemistry, 2011, 2011(30): 6009-6019. |
| [35] | Zhou Y, Debbab A, Má ndi A, Wray V, Schulz B, Mü ller WEG, Kassack M, Lin W, Kurtá n T, Proksch P. Alkaloids from the sponge-associated fungus Aspergillus sp.. European Journal of Organic Chemistry, 2013, 2013(5): 894-906. |
| [36] | Zhou Y, Debbab A, Wray V, Lin W, Schulz B, Trepos R, Pile C, Hellio C, Proksch P, Aly AH. Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp.. Tetrahedron Letters, 2014, 55(17): 2789-2792. |
| [37] | Song F, Ren B, Chen C, Yu K, Liu X, Zhang Y, Yang N, He H, Liu X, Dai H, Zhang L. Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Applied Microbiology and Biotechnology, 2014, 98(8): 3753-3758. |
| [38] | Hiort J, Maksimenka K, Reichert M, Perovic-Ottstadt S, Lin WH, Wray V, Steube K, Schaumann K, Weber H, Proksch P. New natural products from the sponge-derived fungus Aspergillus niger. Journal of Natural Products, 2004, 67(9): 1532-1543. |
| [39] | Ui H, Shiomi K, Yamaguchi Y, Masuma R, Nagamitsu T, Takano D, Sunazuka T, Namikoshi M, Omura S. Nafuredin, a novel inhibitor of NADH-fumarate reductase, produced by Aspergillus niger FT-0554. The Journal of Antibiotics(Tokyo), 2001, 54(3): 234-238. |
| [40] | Parvatkar RR, D’ Souza, Tripathi A. Aspernolides A and B, butenolides from a marine-derived fungus Aspergillus terreus. Phytochemistry, 2009, 70(1): 128-132. |
| [41] | Wei MY, Wang CY, Liu QA, Shao CL, She ZG, Lin YC. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a gorgonian Dichotella gemmacea. Marine Drugs, 2010, 8(4): 941-949. |
| [42] | Shao CL, Wang CY, Wei MY, Gu YC, She ZG, Qian PY, Lin YC. Aspergilones A and B, two benzylazaphilones with an unprecedented carbon skeleton from the gorgonian-derived fungus Aspergillus sp.. Bioorganic and Medicinal Chemistry Letters, 2011, 21(2): 690-693. |
| [43] | Trisuwan K, Rukachaisirikul V, Kaewpet M, Phongpaichit S, Hutadilok-Towatana N, Preedanon S, Sakayaroj J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. Journal of Natural Products, 2011, 74(7): 1663-1667. |
| [44] | Zhuang Y, Teng X, Wang Y, Liu P, Wang H, Li J, Li G, Zhu W. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron, 2011, 67(37): 7085-7089. |
| [45] | Zhuang Y, Teng X, Wang Y, Liu P, Li G, Zhu W. New quinazolinone alkaloids within rare amino acid residue from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Organic Letters, 2011, 13(5): 1130-1133. |
| [46] | Hill RA, Sutherland A. Hot off the press. Natural Products Report, 2011, 28(10): 1621. |
| [47] | Hill RA, Sutherland A. Hot off the press. Natural Products Report, 2011, 28(6): 1031. |
| [48] | Wang L, Zhu W. Versicolactones A and B: total synthesis and structure revision. Tetrahedron Letters, 2013, 54(49): 6729-6731. |
| [49] | Zhang D, Satake M, Fukuzawa S, Sugahara K, Niitsu A, Shirai T, Tachibana K. Two new indole alkaloids, 2-(3, 3-dimethylprop-1-ene)-costaclavine and 2-(3, 3-dimethylprop-1-ene)-epicostaclavine, from the marine-derived fungus Aspergillus fumigatus. Natural Product Communications, 2012, 66(1): 222-226. |
| [50] | Afiyatullov SS, Zhuravleva OI, Chaikina EL, Anisimov MM. A new spirotryprostatin from the marine isolate of the fungus Aspergillus fumigatus. Chemistry of Natural Compounds, 2012, 48(1): 95-98. |
| [51] | Afiyatullov S, Zhuravleva OI, Antonov AS, Kalinovsky AI, Pivkin MV, Menchinskaya ES, Aminin DL. New metabolites from the marine-derived fungus Aspergillus fumigatus. Natural Product Communications, 2012, 7(4): 497-500. |
| [52] | He F, Sun YL, Liu KS, Zhang XY, Qian PY, Wang YF, Qi SH. Indole alkaloids from marine-derived fungus Aspergillus sydowii SCSIO 00305. The Journal of Antibiotics (Tokyo), 2012, 65(2): 109-111. |
| [53] | Zhang D, Fukuzawa S, Satake M, Li X, Kuranaga T, Niitsu A, Yoshizawa K, Tachibana K. Ophiobolin O and 6-epi-ophiobolin O, two new cytotoxic sesterterpenes from the marine derived fungus Aspergillus sp.. Natural Product Communications, 2012, 7(11): 1411-1414. |
| [54] | Yang T, Lu Z, Meng L, Wei S, Hong K, Zhu W, Huang C. The novel agent ophiobolin O induces apoptosis and cell cycle arrest of MCF-7 cells through activation of MAPK signaling pathways. Bioorganic and Medicinal Chemistry Letters, 2012, 22(1): 579-585. |
| [55] | He F, Bao J, Zhang XY, Tu ZC, Shi YM, Qi SH. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. Journal of Natural Products, 2013, 76(6): 1182-1186. |
| [56] | Chen M, Shao CL, Fu XM, Xu RF, Zheng JJ, Zhao DL, She ZG, Wang CY. Bioactive indole alkaloids and phenyl ether derivatives from a marine-derived Aspergillus sp. fungus. Journal of Natural Products, 2013, 76(4): 547-553. |
| [57] | Zheng CJ, Shao CL, Wu LY, Chen M, Wang KL, Zhao DL, Sun XP, Chen GY, Wang CY. Bioactive phenylalanine derivatives and cytochalasins from the soft coral-derived fungus, Aspergillus elegans. Marine Drugs, 2013, 11(6): 2054-2068. |
| [58] | Bao J, Xu XY, Zhang XY, Qi SH. A new macrolide from a marine-derived fungus Aspergillus sp.. Natural Product Communications, 2013, 8(8): 1127-1128. |
| [59] | Liu Z, Xia G, Chen S, Liu Y, Li H, She Z. Eurothiocin A and B, sulfur-containing benzofurans from a soft coral-derived fungus Eurotium rubrum SH-823. Marine Drugs, 2014, 12(6): 3669-3680. |
| [60] | Chen M, Fu X, Kong C, Wang C. Nucleoside derivatives from the marine-derived fungus Aspergillus versicolor. Natural Product Research, 2014, 28(12): 895-900. |
| [61] | Jiang T, Li T, Li J, Fu H, Pei Y, Lin W. Cerebroside analogues from marine-derived fungus Aspergillus flavipes. Journal of Asian Natural Products Research, 2004, 6(4): 249-257. |
| [62] | Snider BB, Zeng H. Total syntheses of (-)-fumiquinazolines A, B, and I. Organic Letters, 2000, 2(25): 4103-4106. |
| [63] | Wang H, Ganesan A. Total synthesis of the fumiquinazoline alkaloids: solution-phase studies1. Journal of Organic Chemistry, 2000, 65(4): 1022-1030. |
| [64] | Takahashi C, Matsushita T, Doi M, Minoura K, Shingu T, Kumeda Y, Numata A. Fumiquinazolines A- G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. Journal of the Chemical Society, Dalton Transactions 1, 1995, (18): 2345-2353. |
| [65] | Yamada T, Imai E, Nakatuji K, Numata A, Tanaka R. Cephalimysin A, a potent cytotoxic metabolite from an Aspergillus species separated from a marine fish. Tetrahedron Letters, 2007, 48(36): 6294-6296. |
| [66] | Yamada T, Kitada H, Kajimoto T, Numata A, Tanaka R. The relationship between the CD cotton effect and the absolute configuration of FD-838 and its seven stereoisomers. Journal of Organic Chemistry, 2010, 75(12): 4146-4153. |
| [67] | Zhu T, Chen Z, Liu P, Wang Y, Xin Z, Zhu W. New rubrolides from the marine-derived fungus Aspergillus terreus OUCMDZ-1925. The Journal of Antibiotics (Tokyo), 2014, 67(4): 315-318. |
| [68] | Tsukamoto S, Hirota H, Imachi M, Fujimuro M, Onuki H, Ohta T, Yokosawa H. Himeic acid A: a new ubiquitin-activating enzyme inhibitor isolated from a marine-derived fungus, Aspergillus sp.. Bioorganic and Medicinal Chemistry Letters, 2005, 15(1): 191-194. |
| [69] | Kuwana T, Miyazaki M, Kato H, Tsukamoto S. Himeic acids E- G, new 4-pyridone derivatives from a culture of Aspergillus sp.. Chemical and Pharmaceutical Bulletin (Tokyo), 2013, 61(1): 105-107. |
| [70] | Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Notoamides A- D: prenylated indole alkaloids isolated from a marine-derived fungus, Aspergillus sp.. Angewandte Chemie International Edition, 2007, 46(13): 2254-2256. |
| [71] | Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. Notoamides F-K, prenylated indole alkaloids isolated from a marine-derived Aspergillus sp.. Journal of Natural Products, 2008, 71(12): 2064-2067. |
| [72] | Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Isolation of notoamide E, a key precursor in the biosynthesis of prenylated indole alkaloids in a marine-derived fungus, Aspergillus sp.. Journal of the American Chemical Society, 2009, 131(11): 3834-3835. |
| [73] | Tsukamoto S, Kawabata T, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Isolation of antipodal (-)-versicolamide B and notoamides L-N from a marine-derived Aspergillus sp.. Organic Letters, 2009, 11(6): 1297-1300. |
| [74] | Tsukamoto S, Umaoka H, Yoshikawa K, Ikeda T, Hirota H. Notoamide O, a structurally unprecedented prenylated indole alkaloid, and notoamides P-R from a marine-derived fungus, Aspergillus sp.. Journal of Natural Products, 2010, 73(8): 1438-1440. |
| [75] | Ding Y, de Wet JR, Cavalcoli J, Li S, Greshock TJ, Miller KA, Finefield JM, Sunderhaus JD, McAfoos TJ, Tsukamoto S, Williams RM, Sherman DH. Genome-based characterization of two prenylation steps in the assembly of the stephacidin and notoamide anticancer agents in a marine-derived Aspergillus sp.. Journal of the American Chemical Society, 2010, 132(36): 12733-12740. |
| [76] | Kitano M, Yamada T, Amagata T, Minoura K, Tanaka R, Numata A. Novel pyridino-α-pyrone sesquiterpene type pileotin produced by a sea urchin-derived Aspergillus sp.. Tetrahedron Letters, 2012, 53(32): 4192-4194. |
| [77] | Nakanishi K, Doi M, Usami Y, Amagata T, Minoura K, Tanaka R, Numata A, Yamada T. Anthcolorins A-F, novel cytotoxic metabolites from a sea urchin-derived Aspergillus versicolor. Tetrahedron, 2013, 69(23): 4617-4623. |
| [78] | Wang R, Liu T, Shen M, Yang M, Feng Q, Tang X, Li X. Spiculisporic acids B- D, three new γ-butenolide derivatives from a sea urchin-derived fungus Aspergillus sp. HDf2. Molecules, 2012, 17(11): 13175-13182. |
| [79] | Jiang W, Ye P, Chen CA, Wang K, Liu P, He S, Wu X, Gan L, Ye Y, Wu B. Two novel hepatocellular carcinoma cycle inhibitory cyclodepsipeptides from a hydrothermal vent crab-associated fungus Aspergillus clavatus C2WU. Marine Drugs, 2013, 11(12): 4761-4772. |
| [80] | Ye P, Shen L, Jiang W, Ye Y, Chen CA, Wu X, Wang K, Wu B. Zn-driven discovery of a hydrothermal vent fungal metabolite clavatustide C, and an experimental study of the anti-cancer mechanism of clavatustide B. Marine Drugs, 2014, 12(6): 3203-3217. |
| [81] | Wang F, Fang Y, Zhu T, Zhang M, Lin A, Gu Q, Zhu W. Seven new prenylated indole diketopiperazine alkaloids from holothurian-derived fungus Aspergillus fumigatus. Tetrahedron, 2008, 64(34): 7986-7991. |
| [82] | Wang F, Li D, Zhu T, Zhang M, Gu Q. Pseurotin A1 and A2, two new 1-oxa-7-azaspiro [4. 4] non-2-ene-4, 6-diones from the holothurian-derived fungus Aspergillus fumigatus WFZ-25. Canadian Journal of Chemistry, 2011, 89(1): 72-76. |
| [83] | Bugni TS, Abbanat D, Bernan VS, Maiese WM, Greenstein M, Van Wagoner RM, Ireland CM. Yanuthones: novel metabolites from a marine isolate of Aspergillus niger. Journal of Organic Chemistry, 2000, 65(21): 7195-7200. |
| [84] | Tsukamoto S, Miura S, Yamashita Y, Ohta T. Aspermytin A: a new neurotrophic polyketide isolated from a marine-derived fungus of the genus Aspergillus. Bioorganic and Medicinal Chemistry Letters, 2004, 14(2): 417-420. |
| [85] | Sun K, Li Y, Guo L, Wang Y, Liu P, Zhu W. Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the Prawn, Penaeus vannamei. Marine Drugs, 2014, 12(7): 3970-3981. |
| [86] | Xu J, Zhao S, Yang X. A new cyclopeptide metabolite of marine gut fungus from Ligia oceanica. Natural Product Research, 2014, 28(13): 994-997. |
| [87] | Xu J, Nakazawa T, Ukai K, Kobayashi H, Mangindaan REP, Wewengkang DS, Rotinsulu H, Namikoshi M. Tetrahydrobostrycin and 1-deoxytetrahydrobostrycin, two new hexahydroanthrone derivatives, from a marine-derived fungus Aspergillus sp.. The Journal of Antibiotics (Tokyo), 2008, 61(7): 415-419. |
| [88] | Rahbæ k L, Christophersen C, Frisvad J, Bengaard HS, Larsen S, Rassing BR. Insulicolide A: a new nitrobenzoyloxy-substituted sesquiterpene from the marine fungus Aspergillus insulicola. Journal of Natural Products, 1997, 60(8): 811-813. |
| [89] | Belofsky GN, Jensen PR, Renner MK, Fenical W. New cytotoxic sesquiterpenoid nitrobenzoyl esters from a marine isolate of the fungus Aspergillus versicolor. Tetrahedron, 1998, 54(9): 1715-1724. |
| [90] | Lorenz P, Jensen PR, Fenical W. Mactanamide, a new fungistatic diketopiperazine produced by a marine Aspergillus sp.. Natural Products Letters, 1998, 12(1): 55-60. |
| [91] | Son BW, Choi JS, Kim JC, Nam KW, Kim D, Chung HY, Kang JS, Choi HD. Parasitenone, a new epoxycyclohexenone related to gabosine from the marine-derived fungus Aspergillus parasiticus. Journal of Natural Products, 2002, 65(5): 794-795. |
| [92] | Lee SM, Li XF, Jiang H, Cheng JG, Seong S, Choi HD, Son BW. Terreusinone, a novel UV-A protecting dipyrroloquinone from the marine algicolous fungus Aspergillus terreus. Tetrahedron Letters, 2003, 44(42): 7707-7710. |
| [93] | Li Y, Li X, Kim S, Kang JS, Choi HD, Rho JR, Son BW. Golmaenone, a new diketopiperazine alkaloid from the marine-derived fungus Aspergillus sp.. Chemical and Pharmaceutical Bulletin (Tokyo), 2004, 52(3): 375-376. |
| [94] | Teuscher F, Lin W, Wray V, Edrada R, Padmakumar K, Proksch P, Ebel R. Two new cyclopentanoids from the endophytic fungus Aspergillus sydowii associated with the marine alga Acanthophora spicifera. Natural Product Communications, 2006, 1(11): 927-933. |
| [95] | Nguyen HP, Zhang D, Lee U, Kang JS, Choi HD, Son BW. Dehydroxychlorofusarielin B, an antibacterial polyoxygenated decalin derivative from the marine-derived fungus Aspergillus sp.. Journal of Natural Products, 2007, 70(7): 1188-1190. |
| [96] | Zhang Y, Li X, Wang B. Nigerasperones A- C, new monomeric and dimeric naphtho-γ-pyrones from a marine alga-derived endophytic fungus Aspergillus niger EN-13. The Journal of Antibiotics (Tokyo), 2007, 60(3): 204-210. |
| [97] | Zhang Y, Wang S, Li XM, Cui CM, Feng C, Wang BG. New sphingolipids with a previously unreported 9-methyl-C20-sphingosine moiety from a marine algous endophytic fungus Aspergillus niger EN-13. Lipids, 2007, 42(8): 759-764. |
| [98] | Zhang Y, Li X, Proksch P, Wang B. Ergosterimide, a new natural diels- alder adduct of a steroid and maleimide in the fungus Aspergillus niger. Steroids, 2007, 72(9): 723-727. |
| [99] | Zhang Y, Li XM, Wang CY. A new naphthoquinoneimine derivative from the marine algal-derived endophytic fungus Aspergillus niger EN-13. Chinese Chemical Letters, 2007, (8): 951-953. |
| [100] | Zhang Y, Li XM, Feng Y, Wang BG. Phenethyl-alpha-pyrone derivatives and cyclodipeptides from a marine algous endophytic fungus Aspergillus niger EN-13. Natural Product Research, 2010, 24(11): 1036-1043. |
| [101] | Lin A, Lu X, Fang Y, Zhu T, Gu Q, Zhu W. Two new 5-hydroxy-2-pyrone derivatives isolated from a marine-derived fungus Aspergillus flavus. The Journal of Antibiotics (Tokyo), 2008, 61(4): 245-249. |
| [102] | Lin A, Du L, Fang Y, Wang F, Zhu T, Gu Q, Zhu W. Iso-α-cyclopiazonic acid, a new natural product isolated from the marine-derived fungus Aspergillus flavus C-F-3. Chemistry of Natural Compounds, 2009, (5): 677-680. |
| [103] | Cui CM, Li XM, Li CS, Sun HF, Gao SS, Wang BG. Benzodiazepine alkaloids from marine-derived endophytic fungus Aspergillus ochraceus. Helvetica Chimica Acta, 2009, 92(7): 1366-1370. |
| [104] | Cui CM, Li XM, Meng L, Li CS, Huang CG, Wang BG. 7-Nor-ergosterolide, a pentalactone-containing norsteroid and related steroids from the marine-derived endophytic Aspergillus ochraceus EN-31. Journal of Natural Products, 2010, 73(11): 1780-1784. |
| [105] | Qiao MF, Ji NY, Liu XH, Li K, Zhu QM, Xue QZ. Indoloditerpenes from an algicolous isolate of Aspergillus oryzae. Bioorganic and Medicinal Chemistry Letters, 2010, 20(19): 5677-5680. |
| [106] | Qiao MF, Ji NY, Liu XH, Li F, Xue QZ. Asporyergosterol, a new steroid from an algicolous isolate of Aspergillus oryzae. Natural Product Communications, 2010, 5(10): 1575-1578. |
| [107] | Izumikawa M, Hashimoto J, Takagi M, Shin-ya K. Isolation of two new terpeptin analogs— JBIR-81 and JBIR-82-from a seaweed-derived fungus, Aspergillus sp. SpD081030G1f1. The Journal of Antibiotics (Tokyo), 2010, 63(7): 389-391. |
| [108] | Yang G, Sandjo L, Yun K, Leutou AS, Kim GD, Choi HD, Kang JS, Hong J, Son BW. Flavusides A and B, antibacterial cerebrosides from the marine-derived fungus Aspergillus flavus. Chemical and Pharmaceutical Bulletin (Tokyo), 2011, (9): 1174-1177. |
| [109] | Qiao MF, Ji NY, Miao FP, Yin XL. Steroids and an oxylipin from an algicolous isolate of Aspergillus flavus. Magnetic Resonance in Chemistry, 2011, 49(6): 366-369. |
| [110] | Zhuravleva OI, Afiyatullov S, Denisenko VA, Ermakova SP, Slinkina NN, Dmitrenok PS, Kim NY. Secondary metabolites from a marine-derived fungus Aspergillus carneus Blochwitz. Phytochemistry, 2012, 80: 123-131. |
| [111] | Zhuravleva OI, Afiyatullov S, Yurchenko EA, Denisenko VA, Kirichuk NN, Dmitrenok PS. New metabolites from the algal associated marine-derived fungus Aspergillus carneus. Natural Product Communications, 2013, 8(8): 1071-1074 |
| [112] | Sun HF, Li XM, Meng L, Cui CM, Gao SS, Li CS, Huang CG, Wang BG. Asperolides A-C, tetranorlabdane diterpenoids from the marine alga-derived endophytic fungus Aspergillus wentii EN-48. Journal of Natural Products, 2012, 75(2): 148-152 |
| [113] | Li X, Li X, Xu G, Li C, Wang B. Antioxidant metabolites from marine alga-derived fungus Aspergillus wentii EN-48. Phytochemistry Letters, 2014, 7: 120-123. |
| [114] | Hawas UW, El-Beih AA, El-Halawany AM. Bioactive anthraquinones from endophytic fungus Aspergillus versicolor isolated from red sea algae. Archives of Pharmacal Research, 2012, 35(10): 1749-1756. |
| [115] | Liu XH, Miao FP, Qiao MF, Cichewicz RH, Ji NY. Terretonin, ophiobolin, and drimane terpenes with absolute configurations from an algicolous Aspergillus ustus. RSC Advances, 2013, 3(2): 588-595. |
| [116] | Miao F, Li X, Liu X, Cichewicz RH, Ji N. Secondary metabolites from an algicolous Aspergillus versicolor strain. Marine Drugs, 2012, 10(1): 131-139. |
| [117] | Liu X, Miao F, Li X, Yin X, Ji N. A new sesquiterpene from an endophytic Aspergillus versicolor strain. Natural Product Communications, 2012, 7(7): 819-820. |
| [118] | Ji N, Liu X, Miao F, Qiao M. Aspeverin, a new alkaloid from an algicolous strain of Aspergillus versicolor. Organic Letters, 2013, 15(10): 2327-2329. |
| [119] | Zhang Y, Li XM, Wang BG. Anthraquinone derivatives produced by marine-derived fungus Aspergillus versicolor EN-7. Bioscience, Biotechnology and Biochemistry, 2012, (9): 1774-1776. |
| [120] | Li Y, Sun K, Wang Y, Fu P, Liu P, Wang C, Zhu W. A cytotoxic pyrrolidinoindoline diketopiperazine dimer from the algal fungus Eurotium herbariorum HT-2. Chinese Chemical Letters, 2013, 24(12): 1049-1052. |
| [121] | Fang W, Lin X, Zhou X, Wan J, Lu X, Yang B, Ai W, Lin J, Zhang T, Tu Z. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. Medicinal Chemistry Communications, 2014, 5(6): 701-705. |
| [122] | Ebada SS, Fischer T, Hamacher A, Du F, Roth YO, Kassack MU, Wang B, Roth EH. Psychrophilin E, a new cyclotripeptide, from co-fermentation of two marine alga-derived fungi of the genus Aspergillus. Natural Product Research, 2014, 28(11): 776-781. |
| [123] | Ogawa A, Murakami C, Kamisuki S, Kuriyama I, Yoshida H, Sugawara F, Mizushina Y. Pseudodeflectusin, a novel isochroman derivative from Aspergillus pseudodeflectus a parasite of the sea weed, Sargassum fusiform, as a selective human cancer cytotoxin. Bioorganic and Medicnal Chemistry Letters, 2004, 14(13): 3539-3543. |
| [124] | Kanoh K, Kohno S, Asari T, Harada T, Katada J, Muramatsu M, Kawashima H, Sekiya H, Uno I. (−)-Phenylahistin: a new mammalian cell cycle inhibitor produced by Aspergillus ustus. Bioorganic and Medicinal Chemistry Letters, 1997, 7(22): 2847-2852. |
| [125] | Couladouros EA, Magos AD. Solid-phase total synthesis of (−)-phenylhistine and (−)-aurantiamine. Synthesis of a diverse dehydro-2, 5-diketopiperazine library. Part II. Molecular Diversity, 2005, 9(1-3): 111-121. |
| [126] | Shangguan N, Joullié MM. Total synthesis of isoroquefortine E and phenylahistin. Tetrahedron Letters, 2009, 50(49): 6755-6757. |
| [127] | Yakushiji F, Tanaka H, Muguruma K, Iwahashi T, Yamazaki Y, Hayashi Y. Prodrug study of plinabulin using a click strategy focused on the effects of a replaceable water-solubilizing moiety. Chemical and Pharmaceutical Bulletin, 2012, 60(7): 877-881. |
| [128] | Mita MM, Spear MA, Yee LK, Mita AC, Heath EI, Papadopoulos KP, Federico KC, Reich SD, Romero O, Malburg L. Phase 1 first-in-human trial of the vascular disrupting agent plinabulin (NPI-2358) in patients with solid tumors or lymphomas. Clinical Cancer Research, 2010, 16(23): 5892-5899. |
| [129] | Millward M, Mainwaring P, Mita A, Federico K, Lloyd GK, Reddinger N, Nawrocki S, Mita M, Spear MA. Phase 1 study of the novel vascular disrupting agent plinabulin (NPI-2358) and docetaxel. Investigational New Drugs, 2012, 30(3): 1065-1073. |
| [130] | Hayashi Y, Takeno H, Chinen T, Muguruma K, Okuyama K, Taguchi A, Takayama K, Yakushiji F, Miura M, Usui T. Development of a new benzophenone-diketopiperazine-type potent anti-microtubule agent possessing a 2-pyridine structure. ACS Medicinal Chemistry Letters, 2014, 5(10): 1094-1098. |
| [131] | Schlingmann G, Taniguchi T, He H, Bigelis R, Yang HY, Koehn FE, Carter GT, Berova N. Reassessing the structure of pyranonigrin. Journal of Natural Products, 2007, 70(7): 1180-1187. |
| [132] | Lin Z, Zhang G, Zhu T, Liu R, Wei H, Gu Q. Bioactive cytochalasins from Aspergillus flavipes, an endophytic fungus associated with the mangrove plant Acanthus ilicifolius. Helvetica Chimica Acta, 2009, 92(8): 1538-1544. |
| [133] | Li D, Li X, Wang B. Natural anthraquinone derivatives from a marine mangrove plant-derived endophytic fungus Eurotium rubrum: structural elucidation and DPPH radical scavenging activity. Journal of Industrial Microbiology and Biotechnology, 2009, 19(7): 675-680. |
| [134] | Huang HB, Feng XJ, Liu L, Chen B, Lu YJ, Ma L, She ZG, Lin YC. Three dimeric naphtho-gamma-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from Pongamia pinnata. Planta Medica, 2010, 76(16): 1888-1891. |
| [135] | Huang HB, Xiao ZE, Feng XJ, Huang CH, Zhu X, Ju JH, Li MF, Lin YC, Liu L, She ZG. Cytotoxic naphtho-g-pyrones from the mangrove endophytic fungus Aspergillus tubingensis (GX1-5E). Helvetica Chimica Acta, 2011, 94(9): 1732-1740. |
| [136] | Liu D, Li XM, Meng L, Li CS, Gao SS, Shang Z, Proksch P, Huang CG, Wang BG. Nigerapyrones A-H, alpha-pyrone derivatives from the marine mangrove-derived endophytic fungus Aspergillus niger MA-132. Journal of Natural Products, 2011, 74(8): 1787-1791. |
| [137] | Song YX, Qiao LT, Wang JJ, Zeng HM, She ZG, Miao CD, Hong K, Gu YC, Liu L, Lin YC. Two new meroterpenes from the mangrove endophytic fungus Aspergillus sp. 085241B. Helvetica Chimica Acta, 2011, 94(10): 1875-1880. |
| [138] | Wang H, Lu Z, Qu H, Liu P, Miao C, Zhu T, Li J, Hong K, Zhu W. Antimicrobial aflatoxins from the marine-derived fungus Aspergillus flavus 092008. Archives of Pharmacal Research, 2012, 35(8): 1387-1392. |
| [139] | Li S, Wei M, Chen G, Lin Y. Two new dihydroisocoumarins from the endophytic fungus Aspergillus sp. collected from the south china sea. Chemistry of Natural Compounds, 2012, 48(3): 371-373. |
| [140] | An C, Li X, Luo H, Li C, Wang M, Xu G, Wang B. 4-phenyl-3, 4-dihydroquinolone derivatives from Aspergillus nidulans MA-143, an endophytic fungus isolated from the mangrove plant Rhizophora stylosa. Journal of Natural Products, 2013, 76(10): 1896-1901. |
| [141] | An C, Li X, Li C, Wang M, Xu G, Wang B. Aniquinazolines A- D, four new quinazolinone alkaloids from marine-derived endophytic fungus Aspergillus nidulans. Marine Drugs, 2013, 11(7): 2682-2694. |
| [142] | Bai Z, Lin X, Wang Y, Wang J, Zhou X, Yang B, Liu J, Yang X, Wang Y, Liu Y. New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia, 2014, 95: 194-202. |
| [143] | Xiao Z, Huang H, Shao C, Xia X, Ma L, Huang X, Lu Y, Lin Y, Long Y, She Z. Asperterpenols A and B, new sesterterpenoids isolated from a mangrove endophytic fungus Aspergillus sp. 085242. Organic Letters, 2013, 15(10): 2522-2525. |
| [144] | Huang X, Huang H, Li H, Sun X, Huang H, Lu Y, Lin Y, Long Y, She Z. Asperterpenoid A, a new sesterterpenoid as an inhibitor of mycobacterium tuberculosi s protein tyrosine phosphatase B from the culture of Aspergillus sp. 16-5c. Organic Letters, 2013, 15(4): 721-723. |
| [145] | Deng C, Liu S, Huang C, Pang J, Lin Y. Secondary metabolites of a mangrove endophytic fungus Aspergillus terreus (No. GX7-3B) from the South China Sea. Marine Drugs, 2013, 11(7): 2616-2624. |
| [146] | Tsuda M, Mugishima T, Komatsu K, Sone T, Tanaka M, Mikami Y, Shiro M, Hirai M, Ohizumi Y, Kobayashi J. Speradine A, a new pentacyclic oxindole alkaloid from a marine-derived fungus Aspergillus tamarii. Tetrahedron, 2003, 59(18): 3227-3230. |
| [147] | Zhang M, Wang W, Fang Y, Zhu T, Gu Q, Zhu W. Cytotoxic alkaloids and antibiotic nordammarane triterpenoids from the marine-derived fungus Aspergillus sydowi. Journal of Natural Products, 2008, 71(6): 985-989. |
| [148] | Capon RJ, Skene C, Stewart M, Ford J, Richard AJ, Williams L, Lacey E, Gill JH, Heiland K, Friedel T. Aspergillicins A- E: five novel depsipeptides from the marine-derived fungus Aspergillus carneus. Organic and Biomolecular Chemistry, 2003, 1(11): 1856-1862. |
| [149] | Smetanina OF, Kalinovskii AI, Khudyakov YV, Moiseenko OP, Pivkin MV, Menzorova NI, Sibirtsev YT, Kuznetsova TA. Metabolites of the marine fungus Asperigillus varians KMM 4630. Chemistry of Natural Compounds, 2005, 41(2): 243-244. |
| [150] | Liu R, Zhu W, Zhang Y, Zhu T, Liu H, Fang Y, Gu Q. A new diphenyl ether from marine-derived fungus Aspergillus sp. BF-2. The Journal of Antibiotics (Tokyo), 2006, 59(6): 362-365. |
| [151] | Boot CM, Gassner NC, Compton JE, Tenney K, Tamble CM, Lokey RS. Pinpointing pseurotins from a marine-derived Aspergillus as tools for chemical genetics using a synthetic lethality yeast screen. Journal of Natural Products, 2007, 70(10): 1672-1675. |
| [152] | Kamigauchi T, Sakazaki R, Nagashima K, Kawamura Y, Yasuda Y, Matsushima K, Tani H, Takahashi Y, Ishii K, Suzuki R, Koizumi K, Nakai H, Ikenishi Y, Terui Y. Terprenins, novel immunosuppressants prduced by Aspergillus candidus. The Journal of Antibiotics (Tokyo), 1998, 51(4): 445-450. |
| [153] | Zhang Y, Zhu T, Fang Y, Liu H, Gu Q, Zhu W. Carbonarones A and B, new bioactive γ-pyrone and α-pyridone derivatives from the marine-derived fungus Aspergillus carbonarius. The Journal of Antibiotics (Tokyo), 2007, 60(2): 153-157. |
| [154] | Zhang Y, Ling S, Fang Y, Zhu T, Gu Q, Zhu W. Isolation, structure elucidation and antimycobacterial properties of dimeric naphtho-γ-pyrones from the marine‐derived fungus Aspergillus carbonarius. Chemistry & Biodiversity, 2008, 5(1): 93-100. |
| [155] | Zhao WY, Zhu TJ, Han XX, Fan GT, Liu HB. A new gliotoxin analogue from a marine-derived fungus Aspergillus fumigatus Fres. Natural Product Research, 2009, 23(3): 203-207. |
| [156] | Zhao WY, Zhu TJ, Fan GT, Liu HB, Fang YC. Three new dioxopiperazine metabolites from a marine-derived fungus Aspergillus fumigatus Fres. Natural Product Research, 2010, 24(10): 953-957. |
| [157] | Fremlin LJ, Piggott AM, Lacey E, Capon RJ. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. Journal of Natural Products, 2009, 72(4): 666-670. |
| [158] | Ren H, Liu R' CL, Zhu T, Zhu W, Gu Q. Two new hetero-spirocyclic gamma-lactam derivatives from marine sediment-derived fungus Aspergillus sydowi D2-6. Archives of Pharmacal Research, 2010, 33(4): 499-502. |
| [159] | Crews MS, Draskovic M, Sohn J, Johnson TA, Tenney K, Valeriote FA, Yao X. Azonazine, a novel dipeptide from a Hawaiian marine sediment-derived fungus, Aspergillus insulicola. Organic Letters, 2010, 12(20): 4458-4461. |
| [160] | Yurchenko AN, Smetanina OF, Kalinovsky AI, Pivkin MV, Dmitrenok PS, Kuznetsova TA. A new meroterpenoid from the marine fungus Aspergillus versicolor (Vuill.) Tirab. Russian Chemical Bulletin, 2010, 59(4): 852-856. |
| [161] | Liu S, Shen Y. A new cyclic peptide from the marine fungal strain Aspergillus sp. AF119. Chemistry of Natural Compounds, 2011, 47(5): 786-788. |
| [162] | Choi EJ, Park JS, Kim YJ, Jung JH, Lee JK, Kwon HC, Yang HO. Apoptosis‐inducing effect of diketopiperazine disulfides produced by Aspergillus sp. KMD 901 isolated from marine sediment on HCT116 colon cancer cell lines. Journal of Applied Microbiology, 2011, 110(1): 304-313. |
| [163] | Liu S, Lu C, Huang J, Shen Y. Three new compounds from the marine fungal strain Aspergillus sp. AF119. Records of Natural Products, 2012, 6(4): 334-338. |
| [164] | Liu S, Zhao B, Lu C, Huang J, Shen Y. Two new p-terphenyl derivatives from the marine fungal strain Aspergillus sp. AF119. Natural Product Communications, 2012, 7(8): 1057-1062. |
| [165] | Sohn J, Oh H. Protulactones A and B: Two new polyketides from the marine-derived fungus Aspergillus sp. SF-5044. Bulletin of the Korean Chemical Society, 2010, 31(6): 1695-1698. |
| [166] | Lee SU, Asami Y, Lee D, Jang JH, Ahn JS, Oh H. Protuboxepins A and B and protubonines A and B from the marine-derived fungus Aspergillus sp. SF-5044. Journal of Natural Products, 2011, 74(5): 1284-1287. |
| [167] | Neff SA, Lee SU, Asami Y, Ahn JS, Oh H, Baltrusaitis J, Gloer JB, Wicklow DT. Aflaquinolones A- G: secondary metabolites from marine and fungicolous isolates of Aspergillus sp.. Journal of Natural Products, 2012, 75(3): 464-472. |
| [168] | Zhou LN, Gao HQ, Cai SX, Zhu TT, Gu QQ, Li DH. Two new cyclic pentapeptides from the marine-derived fungus Aspergillus versicolor. Helvetica Chimica Acta, 2011, 94(6): 1065-1070. |
| [169] | Gao H, Zhou L, Cai S, Zhang G, Zhu T, Gu Q, Li D. Diorcinols BE, new prenylated diphenyl ethers from the marine-derived fungus Aspergillus versicolor ZLN-60. The Journal of Antibiotics (Tokyo), 2013, 66(9): 539-542. |
| [170] | Cai S, Sun S, Zhou H, Kong X, Zhu T, Li D, Gu Q. Prenylated polyhydroxy-p-terphenyls from Aspergillus taichungensis ZHN-7-07. Journal of Natural Products, 2011, 74(5): 1106-1110. |
| [171] | Zhou H, Zhu T, Cai S, Gu Q, Li D. Drimane sesquiterpenoids from the mangrove-derived fungus Aspergillus ustus. Chemical and Pharmaceutical Bulletin (Tokyo), 2011, 59(6): 762-766. |
| [172] | Wang Y. LZ, J Bai LMZ, X Wu LZ, Pei YH. 2,5-diketopiperazines from the marine-derived fungus Aspergillus fumigatus YK-7. Chemistry and Biodiversity, 2012, 9(2): 385-393. |
| [173] | Shen Y, Zou J, Xie D, Ge H, Cao X, Dai J. Butyrolactone and cycloheptanetrione from mangrove-associated fungus Aspergillus terreus. Chemical and Pharmaceutical Bulletin (Tokyo), 2012, 60(11): 1437-1441. |
| [174] | Wu QX, Jin XJ, Draskovic M, Crews MS, Tenney K, Valeriote FA, Yao XJ, Crews P. Unraveling the numerous biosynthetic products of the marine sediment-derived fungus, Aspergillus insulicola. Phytochemistry Letters, 2012, 5(1): 114-117. |
| [175] | Zhuravleva OI, Afiyatullov SS, Vishchuk OS, Denisenko VA, Slinkina NN, Smetanina OF. Decumbenone C, a new cytotoxic decaline derivative from the marine fungus Aspergillus sulphureus KMM 4640. Archives of Pharmacal Research, 2012, 35(10): 1757-1762. |
| [176] | Song F, Liu X, Guo H, Ren B, Chen C, Piggott AM, Yu K, Gao H, Wang Q, Liu M, Liu X, Dai H, Zhang L, Capon RJ. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Organic Letters, 2012, 14(18): 4770-4773. |
| [177] | Lu Z, Wang Y, Miao C, Liu P, Hong K, Zhu W. Sesquiterpenoids and benzofuranoids from the marine-derived fungus Aspergillus ustus 094102. Journal of Natural Products, 2009, 72(10): 1761-1767. |
| [178] | Gao H, Liu W, Zhu T, Mo X, Má ndi A, Kurtá n T, Li J, Ai J, Gu Q, Li D. Diketopiperazine alkaloids from a mangrove rhizosphere soil derived fungus Aspergillus effuses H1-1. Organic and Biomolecular Chemistry 2012, 10(47): 9501-9506. |
| [179] | 刘为忠. 五株海洋真菌次生代谢产物及抗肿瘤活性研究. 中国海洋大学博士学位论文. 2006. |
| [180] | Peng J, Zhang X, Tu Z, Xu X, Qi S. Alkaloids from the deep-sea-derived fungus Aspergillus westerdijkiae DFFSCS013. Journal of Natural Products, 2013, 76(5): 983-987. |
| [181] | Liu X, Song F, Ma L, Chen C, Xiao X, Ren B, Liu X, Dai H, Piggott AM, Av-Gay Y. Sydowiols A- C: Mycobacterium tuberculosis protein tyrosine phosphatase inhibitors from an East China Sea marine-derived fungus, Aspergillus sydowii. Tetrahedron Letters, 2013, 54(45): 6081-6083. |
| [182] | Chen L, Zhang W, Zheng Q. Aculeatusquinones A-D, novel metabolites from the marine-derived fungus Aspergillus aculeatus. Heterocycles, 2013, 87(4): 861-868. |
| [183] | Peng JX, Gao HQ, Li J, Ai J, Geng MY, Zhang GJ, Zhu TT, Gu QQ, Li DH. Prenylated indole diketopiperazines from the marine- derived fungus Aspergillus versicolor. The Journal of Organic Chemistry, 2014, 79(17): 7895-7904. |
| [184] | Hu X, Xia Q, Zhao Y. Speradines B-E, four novel tetracyclic oxindole alkaloids from the marine-derived fungus Aspergillus oryzae. Heterocycles, 2014, 89(7): 1662-1669. |
| [185] | Hu X, Xia Q, Zhao Y, Zheng Q, Liu Q, Chen L, Zhang Q. Speradines F- H, three new oxindole alkaloids from the marine-derived fungus Aspergillus Oryzae. Chemical and Pharmaceutical Bulletin (Tokyo), 2014, 62(9): 942-946. |
| [186] | Dai J, Carté BK, Sidebottom PJ, Sek Yew AL, Ng S, Huang Y, Butler MS. Circumdatin G, a new alkaloid from the fungus Aspergillus ochraceus. Journal of Natural Products, 2001, 64(1): 125-126. |
| [187] | Sun S, Ji C, Gu Q, Li D, Zhu T. Three new polyketides from marine-derived fungus Aspergillus glaucus HB1-19. Journal of Asian Natural Product Research, 2013, 15(9): 956-961. |
| [188] | Du L, Zhu TT, Fang YC, Liu HB, Gu QQ, Zhu WM. Aspergiolide A, a novel anthraquinone derivative with naphtho[1, 2, 3-de]chromene-2, 7-dione skeleton isolated from a marine-derived fungus Aspergillus glaucus. Tetrahedron, 2007, 63(5): 1085-1088. |
| [189] | Zheng J, Zhu H, Hong K, Wang Y, Liu P, Wang X, Peng X, Zhu W. Novel cyclic hexapeptides from marine-derived fungus, Aspergillus sclerotiorum PT06-1. Organic Letters, 2009, 11(22): 5262-5265. |
| [190] | Zheng J, Xu Z, Wang Y, Hong K, Liu P, Zhu W. Cyclic tripeptides from the halotolerant fungus Aspergillus sclerotiorum PT06-1. Journal of Natural Products, 2010, 73(6): 1133-1137. |
| [191] | Wang H, Zheng J, Qu H, Liu P, Wang Y, Zhu W. A new cytotoxic indole-3-ethenamide from the halotolerant fungus Aspergillus sclerotiorum PT06-1. The Journal of Antibiotics (Tokyo), 2011, 64(10): 679-681. |
| [192] | Wang Y, Zheng J, Liu P, Wang W, Zhu W. Three new compounds from Aspergillus terreus PT06-2 grown in a high salt medium. Marine Drugs, 2011, 9(8): 1368-1378. |
| [193] | Cui C. Tryprostatins A and B, novel mammalian cell cycle inhibitors produced by Aspergillus fumigatus. The Journal of Antibiotics (Tokyo), 1995, 48(11): 1382-1384. |
| [194] | Ouyang M, Liu R, Kuo Y. A new cerebroside, asperiamide A, from the marine fungus Asperillus sp. Note. Journal of Asian Natural Product Research, 2005, 7(5): 761-765. |
| [195] | Wu Z, Ouyang M, Su R, Guo Y. Two new cerebrosides and anthraquinone derivatives from the marine fungus Aspergillus niger. Chinese Journal of Chemistry, 2008, 26(4): 759-764. |
| [196] | Wu Z, Ouyang M, Tan Q. New asperxanthone and asperbiphenyl from the marine fungus Aspergillus sp.. Pest Management Science, 2009, 65(1): 60-65. |
| [197] | Dong Y, Cui C, Li C, Hua W, Wu C, Zhu T, Gu Q. Activation of dormant secondary metabolite production by introducing neomycin resistance into the deep-sea fungus, Aspergillus versicolor ZBY-3. Marine Drugs, 2014, 12(8): 4326-4352. |
| [198] | Cai S, Zhu T, Du L, Zhao B, Li D, Gu Q. Sterigmatocystins from the deep-sea-derived fungus Aspergillus versicolor. The Journal of Antibiotics (Tokyo), 2010, 64(2): 193-196. |
| [199] | Kong X, Cai S, Zhu T, Gu Q, Li D, Luan Y. Secondary metabolites of a deep sea derived fungus Aspergillus versicolor CXCTD-06-6a and their bioactivity. Journal of Ocean University of China, 2014, 13(4): 691-695. |
| [200] | Cueto M, Jensen PR, Fenical W. Aspergilloxide, a novel sesterterpene epoxide from a marine-derived fungus of the genus Aspergillus. Organic Letters, 2002, 4(9): 1583-1585. |
| [201] | Ovenden SPB, Sberna G, Tait RM, Wildman HG, Patel R, Li B, Steffy K, Nguyen N, Meurer-Grimes BM. A diketopiperazine dimer from a marine-derived isolate of Aspergillus niger. Journal of Natural Products, 2004, 67(12): 2093-2095. |
| [202] | Yan WP, Song H, Song FH, Guo YS, Wu CH, Her, AS, Pu Y, Wang S, Naowarojna N, Weitz A, Hendrich MP, Costello CE, Zhang LX, Liu PH, Zhang YJ. Endoperoxide formation by an α-ketoglutarate-dependent mononuclear non-haem iron enzyme. Nature, 2015, 527: 539-543. |
| [203] | Xie X, Watanabe K, Wojcicki WA, Wang CC, Tang Y. Biosynthesis of lovastatin analogs with a broadly specific acyltransferase. Chemistry & Biology, 2006, 13(11): 1161-1169. |
| [204] | Mulder KC, Mulinari F, Franco OL, Soares MS, Magalhaes BS, Parachin NS. Lovastatin production: from molecular basis to industrial process optimization. Biotechnology Advances, 2015, 33(6 Pt 1): 648-665. |
| [205] | Xie X, Wong WW, Tang Y. Improving simvastatin bioconversion in Escherichia coli by deletion of bioH. Metabolic Engineering, 2007, 9(4): 379-386. |
| [206] | Xie X, Tang Y. Efficient synthesis of simvastatin using whole-?cell biocatalysis. Applied and Environmental Microbiology, 2007, 73(7): 2054-2060. |
 2016, Vol. 56
2016, Vol. 56




