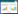中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- Sameh Samir Ali, Rania Al-Tohamy, Jianzhong Sun, Jian Wu, Miao Huang. 2018
- SamehSamir Ali, RaniaAl-Tohamy, 孙建中, 吴建, 黄淼. 2018
- The role of gut symbionts from termites: a unique hidden player from yeasts
- 白蚁肠道共生微生物的作用:酵母, 一个“隐身”的特别菌群
- Acta Microbiologica Sinica, 58(6): 1004-1015
- 微生物学报, 58(6): 1004-1015
-
文章历史
- 收稿日期:2017-12-16
- 修回日期:2018-03-11
- 网络出版日期:2018-03-22
2. Botany Department, Faculty of Science, Tanta University, Tanta 31527, Egypt
2. 坦塔大学科学学院植物学系, 埃及 坦塔 31527
Recently, there has been an increase in the number of studies on the gut symbionts of xylophagous insects like termites, with the objective of determining their role in the metabolic and digestive processes of these insects. Termites thrive in great abundance in the terrestrial ecosystems. Their ability to degrade lignocellulose gives termites an important role in global carbon recycling[1]; and thus, lignocellulose-degrading enzymes from the "termite digestome", both termites and their gut symbionts have many potential bioenergy applications that warrant careful consideration[2]. With the removal of cellulose (74%-99%), hemicellulose (65%-87%) as well as lignin (5%-83%), the digestion of lignocellulose by termites is far more efficient which typically achieving over 95% bioconversion within a day[3]. Termites have developed a unique microflora of archaea, archaezoa, bacteria, yeasts and probably fungi[4]. This specialized microflora enables the termites to efficiently digest lignocellulose and led to a tremendous evolutionary success (Figure 1). Of these symbionts, the anaerobic flagellates produce acetate, CO2 and H2 from cellulose. Acetate is also formed from CO2 and H2 by acetogenic bacteria[5]. Acetate is absorbed by the gut epithelium and functions as a major carbon source of termites. The heterotrophic bacteria fulfill several functions in the hindgut. They fix dinitrogen and recycle the nitrogen of uric acid, maintain a low redox potential, synthesize amino acids, produce acetate and other lower fatty acids, and protect the hindgut from foreign bacteria[6]. Yeasts are single-celled fungi and currently they are becoming the organisms of choice in studies aimed at their potential applications[7]. They are frequent in guts of insects that feed on detritus or wood, and they likely play a part in digestion[8].
|
| Figure 1 Consequences of lignocellulose breakdown by synergistic activities of yeasts and other termites' hindgut symbionts. |
In contrast to previous studies on lignin breakdown, which gave no convincing evidence of microbial degradation of lignin in the termite intestinal tract[9], the recent investigations have shown that the paunch of termites harbors a significant number of yeasts and bacteria with hemicellulose-degrading activities[10-11]. Clearly, between 107 and 5×108 yeast cells were found per mL gut content in Zootermopsis angusticollis and Neotermes castaneus[10]. These yeasts were related to the genera Candida, Pichia, Sporothrix, and Debaromyces. Even though none of these yeasts produced all the required hydrolytic enzymes to completely digest hemicelluloses, they play a significant role in the breakdown of hemicellulose in the termite gut, probably through synergistic activities of their glycolytic enzymes with other gut symbionts (Figure 1). In another study[12], Debaryomyces hansenii and Sporothrix albicans as well as species of Trichosporon and Rhodosporidium could also be found in the hindgut of the termites from families: Mastotermitidae, Hodotermitidae, Kalotermitidae and Rhinotermitidae. Therefore, one may regard the termite gut as a complex ecosystem with free-living and attached microorganisms.
Cellulose and hemicellulose are digested to a large extent during passage of food through the digestive tract by both lower and higher termites, but with different digestive strategies. In the phylogenetically "lower" termites, the gut symbionts include bacteria and protozoa. Many of protozoa are capable of ingesting wood particles and are cellulolytic[13]. However, in the phylogenetically "higher" termites which constitute about 75% of all termite species, the gut microbiota essentially consist of bacteria alone. The confirmed role of bacteria in cellulose digestion is still unclear in lower as well as in higher termites, because animals themselves also produce cellulases[14], and the interesting question: what role do symbionts play? On the other hand, fungi are found in the gut content of termites but do not appear to be involved in cellulose digestion[14] so another interesting questions are: (ⅰ) does the termites produce a cellulase themselves? (ⅱ) does it ingest fungal enzymes? and (ⅲ) what role do yeasts play?
Termite host tissues, via recent transcriptomic efforts, have been shown to produce a litany of cellulases and hemicellulases, as well as lignases, and contribute to lignocellulose degradation[3]. The association with the lignin-degrading fungi enables the fungus-cultivating termites to degrade lignocellulose nearly completely, as reflected in the small volume of their final feces[10]. The key activities attributed to the fungal partner in the mutualistic symbiosis of fungus-cultivating termites are extensive delignification of the substrate and the conversion of plant fiber to fungal biomass[15]. The "acquired enzyme hypothesis" of Martin and Martin[16] was confirmed by evidence for an activity within the gut of fungal cellulases ingested by the termites together with the fungus comb material[17-18]. However, claims that the fungal cellulases are essential for cellulose digestion in the termite guts remain controversial, especially in view of the recently discovered ability of termites to produce their own cellulases[3].
1 Yeast biodiversityThe "yeast" word is broadly used to represent a fungal growth form described by single-cell microorganisms[19]. Still despite their widespread presence, it is thought that only 1% of the diversity of yeast species has been described so far, demonstrating that there is a huge number of undescribed species[20]. At least 1500 yeast species, belonging to the Ascomycota and Basidiomycota phyla, are known[21]. Of Ascomycota, almost 700 yeast species described in 93 genera are called the "true yeasts" and belong to the class Saccharomycetes[19]. These genera include Candida, Saccharomyces, Metschnikowia, Pichia, and Kluveromyces[22]. Cystofilobasidium, Tremella, Cryptococcus, Fellomyces, and Ustilago are yeast genera belonging to Basidiomycota[21, 23].
Most of the known yeast symbionts harbored in the gut of insects belong to Candida, Pichia, Metschnikowia, Pseudozyma, and Cryptococcus[21]. In most cases, these symbionts are grown intercellularly in their host guts[19]. Clearly, true yeasts represent most of the gut symbionts although there are a group of fungal endosymbionts, "yeast-like symbionts" (YLS), that differs from true yeasts. YLS are characterized by evolutionary reduced yeast forms from the Pezizomycotina sub-phylum[19]. However, the diversity and the association between YLS and insects seem to be more limited comparing with true yeasts and insects. To date, the association between YLS and insects have been observed mainly in some species of planthopper, aphid, and anobiid beetles[24]. Overall, it appears mutualistic association between true yeast, YLS and insects which can vary with different taxonomic classification[25]. Generally, the specificity in this association could be correlated to the services provided by the insect to the yeast or vice versa[24].
2 Evolutionary considerations of the intestinal yeastsTermites are well known for their specific prokaryotic and eukaryotic gut symbionts[26]. Despite their wide distribution of the investigated termites in different continents, the majority of yeasts isolated from lower termites comprise a homogeneous assemblage of representatives from the Endomycetales[27]. Based on a genotypic characterization, Trichosporon mycotoxinivorans sp. nov., was the only Basidiomycetous yeast isolated from lower termites. This yeast species is useful in biological detoxification of various mycotoxins. Additionally, a highly symbiotic yeast consortium in the hindgut of the Pachyiulus flavipes was detected[28]. The yeast consortia consist almost exclusively of DTZ-group of Ascomycetes identified to be Debaryomyces hansenii, Torulaspora delbrueckii, and Zygowilliopsis californica[29]. Beside the DTZ-group, other yeast species were minor components in the excrement, and include Pichia membranaefaciens, Trichosporon cutaneum, and Geotrichum candidum. The cell walls of such yeasts probably resist the enzymatic attack in the digestive tract of the insects[30]. In contrast to lower termites, the symbiotic Basidiomycetous yeast species were commonly detected in the hindgut of the higher termite Odontodermes obesus. These species contained xylose in their cell walls and identified to the genera Tremellaand Filobasidium[27].
Based on the 18S rDNA sequences and fossil record, Berbee and Taylor[32] reported that the Endomycetales are a sister group from the filamentous Ascomycetes. Considering a new concept of the carbohydrate composition of yeast cells, sexuality and mating type evolution, Prillinger et al.[31] interpreted the Endomycetales as primitive fungi which have lost their filamentous stage based on the extinction of the hosts[33]. However, arguments to the later interpretation were added by Kurtzman[34]. The presence of Endomycetes yeast symbionts representing the mannose-glucose carbohydrate pattern in lower termite documents that the "Saccharomyces type" is most probably ancestral to the Ustilago, Microbotryum, Protomyces, and Tremella type[31].
Although intimate associations are known between filamentous Ascomycetes or Agaricales and higher termites, there is no information with respect to the yeast mycoflora in termites and its usefulness to trace the phylogeny of Ascomycetous and Basidiomycetous yeasts. If the speculation of Martynov[35] about the evolution of termites is correct, coevolution between termites and yeasts may be extremely useful to trace the phylogeny of filamentous Ascomycetous and Basidiomycetous yeasts[30]. Based on the study of Prillinger et al.[27] about yeast symbionts in the hindgut of termites, it may be concluded that an amalgamation of information obtained from symbiotic associations, ribosomal DNA sequencing, yeast cell wall sugars, fermentation and the fossil record might be a more reliable indicator for fungal phylogeny than evolutionary trees based on ribosomal DNA sequencing alone to trace fungal phylogeny back to early Paleozoic periods[30].
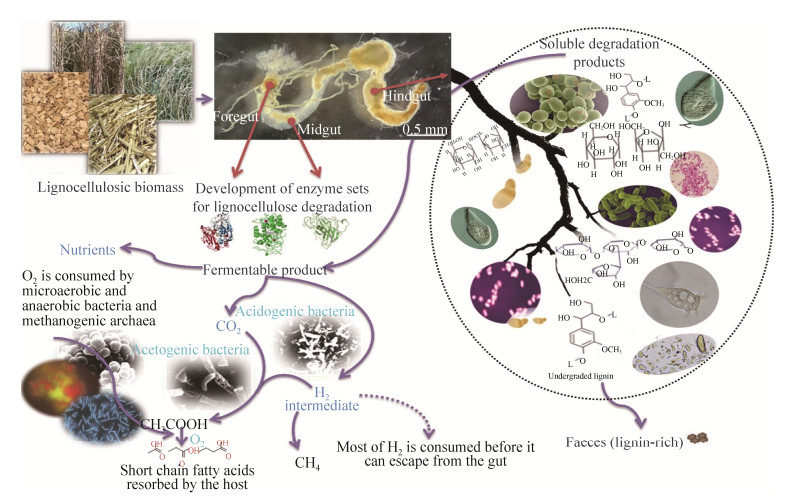
3 The possible benefits from the yeast-termite association 3.1 Benefits for the yeastsTermites can provide yeasts with many benefits (Figure 2). The survival of yeast symbionts is widely demonstrated and is expected to be similar for bacterial symbionts[8]. Generally, it is considered that symbionts are, first, acquired by ingestion. Then, they colonized the insect guts, and finally being released from feces for dispersion of microorganisms. Depending on the insect behavior, yeast cells can be inoculated in eggs by females or they can be re-ingested by colonization of food sources or through trophallaxis and coprophagy[8].
|
| Figure 2 Symbiotic associations between termites and their yeast symbionts. |
Contrary to filamentous fungi, yeasts elaborate spore cell types are able to survive numerous extreme environmental conditions in a laboratory experiment, such as high and low extremes of pH, high salt concentrations, heat shocks, growth at 42 ℃, and ether vapor[36]. These characters demonstrated that spores are more adapted than vegetative cells to endure environmental harsh conditions[37]. The cell wall structure of the spore confers their resistance to stresses associated with digestion and allowing the yeasts to be dispersed by oviposition or insect feeding[38]. Another benefit to yeasts after their consumption by termites is the putative role of digestion in the promotion of outbreeding[37]. This role is fundamental to increase the probability of adaptation and to maintain genetic variation among the microbial progeny. Frequently, inbreeding between the four ascospores housed in the same ascus is observed during sexual reproduction of yeasts. To release these ascospores and avoid inbreeding, enzymes are required to break up the ascus. It has been proposed that the host enzymes of termite guts accomplish this function by breaking down the haploid ascospore tetrads and promoting outbreeding[39].
3.2 Benefits for the termitesYeast symbionts can also provide insects with many benefits (Figure 2). Vage et al.[19] reported that the role of yeasts in insect nutrition is very important, where insect performance would decrease due to the absence of yeast symbionts. Yeasts are sources of B3 and B5 vitamins, amino acids, proteins, and trace metals that could be easily assimilated through simple digestion. In addition, yeast cells contain about 8.5% nitrogen by dry weight, thus insect feeding on yeasts represents a better nitrogen source than the plant tissue itself[19, 40]. Becher et al.[41] reported the nutritional importance of yeasts for Drosophila flies during egg maturation and larval development due to sufficient amounts of nitrogen, vitamins, lipids that yeasts provide. Additionally, previous studies reported the nutritional role of nitrogen, vitamins, essential amino acids, and sterols provided by the YLS on Symbiotaphrina and anobiid beetles[42-43]. Furthermore, the role of yeast symbionts as facilitators of insect host nutrition has been demonstrated[19, 40]. These yeasts play a major role in the production of digestive enzymes that degrade plant lignocellulose into molecules with nutritional value for herbivores. Different types of enzymes are produced by termite-associated yeasts including peptidases, lipases and hydrolases. On the other hand, yeast symbionts in the Lasioderma serricorne, Ernobius mollis and Xestobium piumbeum beetles produce glucosidases lipases, phosphatases and trypsin in order to degrade cellobiose[44]. Recently, research on the role of yeast enzymes has focused on the degradation of complex polymers into simple sugars that the termite can absorb directly. True yeast species of the genus Candida have the ability to degrade wood components such as cellulose, glucosides, and pectin. Therefore, wood-feeding termites might rely on their associated yeast species to degrade cellulose, and their symbionts produce glucosidases and pectinases[45].
3.3 Some roles of the symbiotic yeasts isolated from the termite guts and their potential applicationsTermite gut symbionts might be interesting due to their pronounced solvent tolerance or may be a future source of industrially useful solvent-tolerant enzymes. In addition these microbiota may detoxify allelochemicals or may be responsible for the biosynthesis of essential compounds such as vitamins, sterols, or nitrogen-containing constituents. Generally these microorganisms may be the source of novel metabolic capabilities such as defense substances and antibiotics or may fixate atmospheric nitrogen. It has been known for many years that enzymes in the gut which originate from ingested fungal tissue or gut microbes may mediate cellulolysis. The data on yeasts in the termite guts are scarce. Nevertheless, the first investigations showed that the yeast isolates occur at high numbers in the guts of lower and higher termites of different systematic positions. Since they have been constantly isolated, they belong most probably to the autochthonous symbiotic microbiota of the paunch. They produce cellulolytic and hemicellulolytic enzymes and may play a significant role in the degradation of lignocellulose besides the protists, which thrive only in lower termites and bacteria[46].
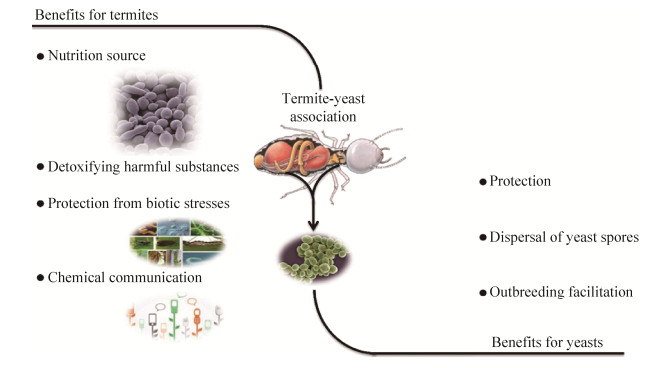
Termites play a major role in the recycling of photosynthetically fixed carbon. With the aid of their gut symbionts they degrade to a high extent the wood constituents cellulose and hemicellulose. Nevertheless, the microbial species involved in the degradation of cellulose and hemicelluloses have been yet poorly defined before the investigations of Schäfer et al.[10] and Wenzel et al.[47]. They identified different yeast strains with cellulose- or xylan-degrading capability. A series of different hemicellulose-degrading yeasts were isolated for the first time from the termite gut[10]. Some of yeast species; namely Candida blankii, Candida edax, Pichia pini, Sporothrix schenkii and Debaryomyces hansenii var fabryii isolated from the lower termites Mastotermis darwiniensis, Neotermes jouteli, Reticulitermes santonensis, Zootermopsis angusticollis and Zootermopsis nevadensis produced clear zones on agar plates containing insoluble xylan (oat spelt) or dye-linked xylan or solubilized xylan (oat spelt) in liquid media[27]. Reports on cellulase production by yeast wild-type strains are rare[48]. Some of the yeast isolates from termites produced hemicellulases[10] and some were also able to degrade cellulose[47]. Some cellulolytic yeast strains, Sporothrix schenkii and Trichosporon sp. were isolated from Zootermopsis nevadensis and Mastotermes darwiniensis, respectively[27]. From a biotechnological perspective, termite gut yeasts constitute promising and mostly untapped sources for potential applications in industrial processes, agriculture, medicine, and bioremediation (Figure 3). A number of approaches based on yeasts from termite guts have been successfully implemented (Table 1).
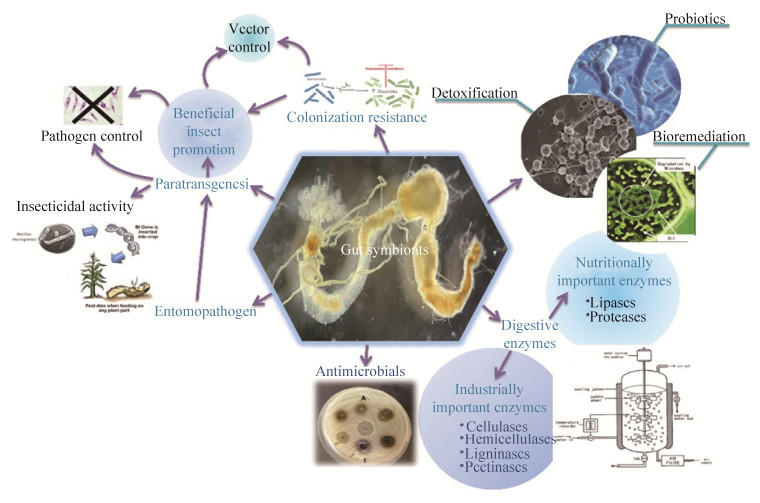
|
| Figure 3 A schematic biotechnological applications potentially for yeasts-associated termites. |
| Application | Importance | Example | Reference |
| Digestive enzymes | Biotechnologically relevant digestive enzymes produced by symbiotic yeasts in termites to overcome the host's nutritional limitations. | *Novel yeasts with potential xylanase activity have been isolated from termites, including Candida pseudorhagii sp. nov. strain SSA-1542T, Hamamotoa lignophila sp. nov. strain SSA-1576T, Meyerozyma guilliermondii sp. nov. strain SSA-1522T, and Sugiyamaella sp.1 nov. strain SSA-1592T from Reticulitermes chinenesis. | [11] |
| *Termite guts of Mastotermes darwiniensis and Odontotermes obesus revealed novel yeast species such as Sugiyamaella mastotermitis sp. nov. strain MD39VT and Papiliotrema odontotermitis f.a. sp. nov. strain OO5T for xylanase production, hydrolysis of methylumbelliferyl β-xylobiose, hydrolysis of methylumbelliferyl β-xylotriose, and production of vitamins. | [49] | ||
| *Cellulases and ligninases production by novel yeast species; namely Meyerozyma guilliermondii sp. nov. strain SSA-1543T and Starmera dryadoides Sp. nov. strain SSA-1549T were detected in Coptotermes formosanus. *Several wood-feeding termites host symbiotic soft-rot fungi in their guts, either to degrade lignin itself or to enable efficient utilization of cellulose as a source of carbon and energy. | [50] | ||
| Paratransgenesis | Using genetically engineered microorganisms to express and deliver gene products in a host organism. | *Genetically engineered yeast that expresses a synthetic protozoacidal lytic peptide (Hecate) coupled to a ligand that specifically binds the lytic peptide to protozoa. When lytic peptide expressing yeast was fed to Formosan subterranean termites in a bait, workers lost all their gut protozoa within three weeks and the termite lab colonies died within two weeks thereafter. | [51] |
| Bioremediation | Transformation, elimination, or attenuation of polluting substances by the use of biological processes. | *Melittin, a natural lytic peptide, expressing yeast did kill protozoa in the termite gut within 56 days of exposure. | [52] |
| *The two ligninolytic yeast strains, Debaryomyces hansenii and Sporothrix albicans, were found in the hindgut of the lower termites Mastotermes darwiniensis, Zootermopsis angusticollis, Zootermopsis nevadensis, Neotermes jouteli, Reticulitermes santonensis, and Heterotermes indicola had a high ability for phenol removal. | [53] | ||
| *Lignin-modifying enzymes have long been used in palm oil mill effluent (POME) treatment to remove the dark brown color resulting from phenolic contamination. This study investigated a cost-effective industrial application method for optimizing phenol removal from POME using the termite-associated yeast Galactomyces reessii obtained from the subterranean termite under laboratory conditions. | [54] | ||
| Antimicrobial activity | Termite-associated yeasts present promising sources of novel antimicrobial secondary metabolites that find important applications in agriculture and human medicines. | Candida lusitaniae strain TERM73 obtained from the termite gut, Cornitermes cumulans was capable of efficient silver/silver chloride nanoparticles (Ag/AgCl-NPs) production. Exposure of Gram-positive Staphylococcus aureus and Gram-negative Klebsiella pneumoniae cultures to Ag/AgCl-NPs led to a strong growth inhibition with a strong potential for use in the biotechnology industry, particularly for biomedical applications. | [49] |
| Detoxification | Detoxifying enzymes providing resistance against toxic natural products as well as noxious compounds from human activities. | The newly yeast strain, Trichosporon mycotoxinivorans sp. nov., isolated from the hindgut of the lower termite Mastotermes darwiniensis had potential application in biological detoxification of various mycotoxins such as ochratoxin A and zearalenone. Therefore this strain can be used for the deactivation of the respective mycotoxins in animal feeds. | [28] |
| Beneficial insect promotion | Development of new biological control strategies to manage pests. | A survey of the mycoflora associated with Reticulitermes flavipes subterranean termite suggests that interactions among fungi may suppress pathogenic effects and promote termite survival. | [55] |
4 Conclusion
Termite gut symbionts, including other insect gut symbionts, constitute a rich and mostly untapped source of bioactive small molecules as well as a variety of digestive enzymes for potential biotechnological values. In addition to exploiting their metabolic capabilities, it has been suggested that the main roles offered by insect gut symbionts are recognized for a nutritional contribution to their diets, promote insect health as well as to suppress pathogens that might hamper the insect development. Consequently, the study of how insects detect microbes has been focused on insects that depend on microorganisms for their dietary requirements. For this reason in the case of yeast-insect symbiosis, most of the available knowledge was derived from the beetles and Drosophilids, while other insects, such as termites, have been greatly ignored. Hence, the study of termites and the association with their yeast symbionts not only promise to uncover interesting new yeast symbionts but may also prove valuable in the continued efforts to find new sources of biotechnologically important molecules and enzymes. Specifically, targeted searches for compounds with particular application values, such as antimicrobial agents, detoxifying enzymes, industrial enzymes (e.g., xylanases, ligninases, cellulases, etc.) may benefit from being guided by the knowledge on the ecology of insect-symbiont interactions, which would have a potential to predict a promising system for future exploration.
| [1] | Scharf ME, Tartar A. Termite digestomes as sources for novel lignocellulases. Biofuels, Bioproducts and Biorefining, 2008, 2(6): 540-552. DOI:10.1002/bbb.v2:6 |
| [2] | Brune A. Symbiotic digestion of lignocellulose in termite guts. Nature Reviews Microbiology, 2014, 12(3): 168-180. DOI:10.1038/nrmicro3182 |
| [3] | Sun JZ, Ding SY, Peterson DJ. Biological conversion of biomass for fuels and chemicals:Explorations from natural utilization systems. Cambridge, UK: Royal Society of Chemistry, 2014. |
| [4] | Sun JZ, Scharf ME. Exploring and integrating cellulolytic systems of insects to advance biofuel technology. Insect Science, 2010, 17(3): 163-165. DOI:10.1111/ins.2010.17.issue-3 |
| [5] | Brauman A, Kane MD, Labat M, Breznak JA. Genesis of acetate and methane by gut bacteria of nutritionally diverse termites. Science, 1992, 257(5075): 1384-1387. DOI:10.1126/science.257.5075.1384 |
| [6] | Breznak JA. Intestinal microbiota of termites and other xylophagous insects. Annual Review of Microbiology, 1982, 36(1): 323-343. DOI:10.1146/annurev.mi.36.100182.001543 |
| [7] | Ali SS, Sun JZ. Physico-chemical pretreatment and fungal biotreatment for park wastes and cattle dung for biogas production. SpringerPlus, 2015, 4: 712. DOI:10.1186/s40064-015-1466-9 |
| [8] | Engel P, Moran NA. The gut microbiota of insects-diversity in structure and function. FEMS Microbiology Reviews, 2013, 37(5): 699-735. DOI:10.1111/1574-6976.12025 |
| [9] | Kuhnigk T, Borst EM, Ritter A, Kämpfer P, Graf A, Hertel H, König H. Degradation of lignin monomers by the hindgut flora of xylophagous termites. Systematic and Applied Microbiology, 1994, 17(1): 76-85. DOI:10.1016/S0723-2020(11)80034-2 |
| [10] | Schäfer A, Konrad R, Kuhnigk T, Kämpfer P, Hertel H, König H. Hemicellulose-degrading bacteria and yeasts from the termite gut. Journal of Applied Bacteriology, 1996, 80(5): 471-478. DOI:10.1111/jam.1996.80.issue-5 |
| [11] | Ali SS, Wu J, Xie RR, Zhou F, Sun JZ, Huang M. Screening and characterizing of xylanolytic and xylose-fermenting yeasts isolated from the wood-feeding termite, Reticulitermes chinensis. PLoS One, 2017, 12(7): e0181141. DOI:10.1371/journal.pone.0181141 |
| [12] | Prillinger H, König H. The intestinal yeasts//König H, Varma A. Intestinal microorganisms of termites and other invertebrates. Berlin, Heidelberg: Springer-Verlag, 2006: 319-334. |
| [13] | Brune A. Symbiotic associations between termites and prokaryotes//Rosenberg E, DeLong EF, Lory S, Stackebrandt E, Thompson F. The prokaryotes. Berlin: Springer, 2013: 545-577. |
| [14] | Slaytor M. Cellulose digestion in termites and cockroaches:what role do symbionts play?. Comparative Biochemistry and Physiology Part B:Comparative Biochemistry, 1992, 103(4): 775-784. DOI:10.1016/0305-0491(92)90194-V |
| [15] | Rouland-Lefèvre C. Symbiosis with fungi//Abe T, Bignell DE, Higashi M. Termites: evolution, sociality, symbiosis, ecology. Dordrecht: Springer, 2000: 289-306. |
| [16] | Martin MM, Martin JS. Cellulose digestion in the midgut of the fungus-growing termite Macrotermes natalensis:the role of acquired digestive enzymes. Science, 1978, 199(4336): 1453-1455. DOI:10.1126/science.199.4336.1453 |
| [17] | Tokuda G, Lo N, Watanabe H, Arakawa G, Matsumoto T, Noda H. Major alteration of the expression site of endogenous cellulases in members of an apical termite lineage. Molecular Ecology, 2004, 13(10): 3219-3228. DOI:10.1111/j.1365-294X.2004.02276.x |
| [18] | Katoh H, Miura T, Maekawa K, Shinzato N, Matsumoto T. Genetic variation of symbiotic fungi cultivated by the macrotermitine termite Odontotermes formosanus (Isoptera:Termitidae) in the Ryukyu Archipelago. Molecular Ecology, 2002, 11(8): 1565-1572. DOI:10.1046/j.1365-294X.2002.01535.x |
| [19] | Vega FE, Blackburn MB, Kurtzman CP, Dowd PF. Identification of a coffee berry borer-associated yeast:Does it break down caffeine?. Entomologia Experimentalis et Applicata, 2003, 107(1): 19-24. DOI:10.1046/j.1570-7458.2003.00034.x |
| [20] | Kurtzman CP, Fell JW. Yeast systematics and phylogeny-implications of molecular identification methods for studies in ecology//Péter G, Rosa C. Biodiversity and Ecophysiology of Yeasts. Berlin, Heidelberg: Springer, 2006: 11-30. |
| [21] | Urubschurov V, Janczyk P. Biodiversity of yeasts in the gastrointestinal ecosystem with emphasis on its importance for the host//Oscar G, Gianfranco V. The dynamical processes of biodiversity-case studies of evolution and spatial distribution. Rijeka, Croatia: InTech, 2011: 277-302. |
| [22] | Nguyen NH, Suh SO, Blackwell M. Five novel Candida species in insect-associated yeast clades isolated from Neuroptera and other insects. Mycologia, 2007, 99(6): 842-858. DOI:10.1080/15572536.2007.11832516 |
| [23] | Landell MF, Inácio J, Fonseca Á, Vainstein MH, Valente P. Cryptococcus bromeliarum sp. nov., an orange-coloured basidiomycetous yeast isolated from bromeliads in Brazil. International Journal of Systematic and Evolutionary Microbiology, 2009, 59(4): 910-913. DOI:10.1099/ijs.0.005652-0 |
| [24] | Sasaki T, Kawamura M, Ishikawa H. Nitrogen recycling in the brown planthopper, Nilaparvata lugens:Involvement of yeast-like endosymbionts in uric acid metabolism. Journal of Insect Physiology, 1996, 42(2): 125-129. DOI:10.1016/0022-1910(95)00086-0 |
| [25] | Suh SO, Noda H, Blackwell M. Insect symbiosis:derivation of yeast-like endosymbionts within an entomopathogenic filamentous lineage. Molecular Biology and Evolution, 2001, 18(6): 995-1000. DOI:10.1093/oxfordjournals.molbev.a003901 |
| [26] | König H, Fröhlich J, Berchtold M, Wenzel M. Diversity and microhabitats of the hindgut flora of termites//Recent research developments in microbiology. Trivandrum:Research Signpost, 2002, 6: 125-156. |
| [27] | Prillinger H, Altenbuchner J, Laaser G, Dörfler C. Yeasts isolated from Homobasidiomycetes (Asterophora, Collybia):New aspects for sexuality, taxonomy, and speciation. Experimental Mycology, 1993, 17(1): 24-45. DOI:10.1006/emyc.1993.1003 |
| [28] | Molnár O, Schatzmayr G, Fuchs E, Prillinger H. Trichosporon mycotoxinivorans sp. nov., a new yeast species useful in biological detoxification of various mycotoxins. Systematic and Applied Microbiology, 2004, 27(6): 661-671. DOI:10.1078/0723202042369947 |
| [29] | Byzov BA, Thanh VN, Babjeva IP. Interrelationships between yeasts and soil diplopods. Soil Biology and Biochemistry, 1993, 25(8): 1119-1126. DOI:10.1016/0038-0717(93)90160-D |
| [30] | Brune A. Methanogens in the digestive tract of termites//Hackstein JHP. (Endo)symbiotic methanogenic archaea. Berlin: Springer, 2010: 81-100. |
| [31] | Zhao JH, Bai FY, Guo LD, Jia JH. Rhodotorula pinicola sp. nov., a basidiomycetous yeast species isolated from xylem of pine twigs. FEMS Yeast Research, 2002, 2(2): 159-163. |
| [32] | Berbee ML, Taylor JW. Dating the evolutionary radiations of the true fungi. Canadian Journal of Botany, 1993, 71(8): 1114-1127. DOI:10.1139/b93-131 |
| [33] | Pant NC, Fraenkel G. Studies on the symbiotic yeasts of two insect species, Lasioderma serricorne F. and Stegobium paniceum L. The Biological Bulletin, 1954, 107(3): 420-432. DOI:10.2307/1538590 |
| [34] | Kurtzman CP. Systematics of the ascomycetous yeasts assessed from ribosomal RNA sequence divergence. Antonie van Leeuwenhoek, 1993, 63(2): 165-174. DOI:10.1007/BF00872391 |
| [35] | Donovan SE, Jones DT, Sands WA, Eggleton P. Morphological phylogenetics of termites (Isoptera). Biological Journal of the Linnean Society, 2000, 70(3): 467-513. DOI:10.1111/bij.2000.70.issue-3 |
| [36] | Messner R, Prillinger H, Ibl M, Himmler G. Sequences of ribosomal genes and internal transcribed spacers move three plant parasitic fungi, Eremothecium ashbyi, Ashbya gossypii, and Nematospora coryli, towards Saccharomyces cerevisiae. The Journal of General and Applied Microbiology, 1995, 41(1): 31-42. DOI:10.2323/jgam.41.31 |
| [37] | Carlile MJ, Watkinson SC, Gooday GW. The fungi. San Diego, London: Academic, 2001. |
| [38] | Suh SO, McHugh JV, Pollock DD, Blackwell M. The beetle gut:a hyperdiverse source of novel yeasts. Mycological Research, 2005, 109(3): 261-265. DOI:10.1017/S0953756205002388 |
| [39] | Coluccio AE, Rodriguez RK, Kernan MJ, Neiman AM. The yeast spore wall enables spores to survive passage through the digestive tract of Drosophila. PLoS One, 2008, 3(8): e2873. DOI:10.1371/journal.pone.0002873 |
| [40] | Reuter M, Bell G, Greig D. Increased outbreeding in yeast in response to dispersal by an insect vector. Current Biology, 2007, 17(3): R81-R83. DOI:10.1016/j.cub.2006.11.059 |
| [41] | Becher PG, Flick G, Rozpędowska E, Schmidt A, Hagman A, Lebreton S, Larsson MC, Hansson BS, Piškur J, Witzgall P, Bengtsson M. Yeast, not fruit volatiles mediate Drosophila melanogaster attraction, oviposition and development. Functional Ecology, 2012, 26(4): 822-828. DOI:10.1111/fec.2012.26.issue-4 |
| [42] | Gibson CM, Hunter MS. Extraordinarily widespread and fantastically complex:comparative biology of endosymbiotic bacterial and fungal mutualists of insects. Ecology Letters, 2010, 13(2): 223-234. DOI:10.1111/ele.2010.13.issue-2 |
| [43] | Bismanis JE. Endosymbionts of Sitodrepa panicea. Canadian Journal of Microbiology, 1976, 22(10): 1415-1424. DOI:10.1139/m76-210 |
| [44] | Jurzitza G. Über Isolierung, Kultur und Taxonomie einiger Anobiidensymbionten (Insecta, Coleoptera). Archiv für Mikrobiologie, 1970, 72(3): 203-222. DOI:10.1007/BF00412173 |
| [45] | Shen SK, Dowd PF. Detoxification spectrum of the cigarette beetle symbiont Symbiotaphrina kochii in culture. Entomologia Experimentalis et Applicata, 1991, 60(1): 51-59. DOI:10.1111/eea.1991.60.issue-1 |
| [46] | Jermy T. Evolution of insect/host plant relationships. The American Naturalist, 1984, 124(5): 609-630. DOI:10.1086/284302 |
| [47] | Wenzel M, Schonig I, Berchtold M, Kanipfer P, Konig H. Aerobic and facultatively anaerobic cellulolytic bacteria from the gut of the termite Zootermopsis angusticollis. Journal of Applied Microbiology, 2002, 92(1): 32-40. DOI:10.1046/j.1365-2672.2002.01502.x |
| [48] | Chararas C, Pignal MC, Vodjdani G, Bourgeay-Causse M. Glycosidases and B group vitamins produced by six yeast strains from the digestive tract of Phoracantha semipunctata larvae and their role in the insect development. Mycopathologia, 1983, 83(1): 9-15. DOI:10.1007/BF00437405 |
| [49] | Eugênio M, Müller N, Frasés S, Almeida-Paes R, Lima LMTR, Lemgruber L, Farina M, de Souza W, Sant'Anna C. Yeast-derived biosynthesis of silver/silver chloride nanoparticles and their antiproliferative activity against bacteria. RSC Advances, 2016, 6(12): 9893-9904. DOI:10.1039/C5RA22727E |
| [50] | Hyodo F, Tayasu I, Inoue T, Azuma JI, Kudo T, Abe T. Differential role of symbiotic fungi in lignin degradation and food provision for fungus-growing termites (Macrotermitinae:Isoptera). Functional Ecology, 2003, 17(2): 186-193. DOI:10.1046/j.1365-2435.2003.00718.x |
| [51] | Sethi A, Delatte J, Foil L, Husseneder C. Protozoacidal trojan-horse:Use of a ligand-lytic peptide for selective destruction of symbiotic protozoa within termite guts. PLoS One, 2014, 9(9): e106199. DOI:10.1371/journal.pone.0106199 |
| [52] | Husseneder C, Donaldson JR, Foil LD. Genetically engineered yeast expressing a lytic peptide from Bee Venom (Melittin) kills symbiotic protozoa in the gut of Formosan subterranean termites. PLoS One, 2016, 11(3): e0151675. DOI:10.1371/journal.pone.0151675 |
| [53] | Prillinger H, Messnge R, König H, Bauer R, Lopandic K, Molnar O, Dangel P, Welgang F, Kirisits T, Nakasse T, Sigler L. Yeasts associated with termites:A phenotypic and genotypic characterization and use of coevolution for dating evolutionary radiations in asco-and basidiomycetes. Applied and Environmental Microbiology, 1996, 19(2): 265-283. |
| [54] | Chaijak P, Lertworapreecha M, Sukkasem C. Phenol removal from palm oil mill effluent using Galactomyces reessii termite-associated yeast. Polish Journal of Environmental Studies, 2018, 27(1): 39-44. DOI:10.15244/pjoes/75205 |
| [55] | Zoberi MH, Grace JK. Fungi associated with the subterranean termite Reticulitermes flavipes in Ontario. Mycologia, 1990, 82(3): 289-294. DOI:10.2307/3759899 |
 2018, Vol. 58
2018, Vol. 58