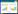中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 郑金伟, 袁权, 夏宁邵. 2019
- Jinwei Zheng, Quan Yuan, Ningshao Xia. 2019
- 慢性乙型肝炎潜在治疗靶点和新药研发进展
- Novel potential treatments for chronic hepatitis B virus infections
- 微生物学报, 59(8): 1437-1451
- Acta Microbiologica Sinica, 59(8): 1437-1451
-
文章历史
- 收稿日期:2018-09-29
- 修回日期:2018-12-14
- 网络出版日期:2019-03-15
2. 厦门大学生命科学学院, 国家传染病诊断试剂与疫苗工程技术中心, 福建 厦门 361102
2. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Life Sciences, Xiamen University, Xiamen 361102, Fujian Province, China
乙型肝炎病毒(hepatitis B virus,HBV)的慢性感染仍是全球主要公共卫生问题之一。根据世界卫生组织报道,目前全球有超过2.4亿的慢性HBV感染者,如未及时得到有效治疗,其中约25%的患者将发展为肝癌、肝硬化等终末期肝病并因此死亡[1]。目前临床上认为治疗慢性HBV感染患者的理想终点是使患者达到HBV表面抗原阴转(HBsAg loss)或血清学转换(HBsAg SR)[2-3]。然而,目前的治疗药物仅局限于核苷(酸)类似物(nucleotide/nucleoside analogues,NAs)和干扰素α两大类。NAs具有良好的耐受性,可以有效抑制HBV复制和控制肝病发生,但是对HBV的抑制作用是可逆的,停药后短期内复发的可能性非常大,长期治疗后HBsAg的清除率小于5%且无法根除cccDNA[4-5]。另外,干扰素长期使用可以一定程度地抑制HBV DNA的复制,抑制cccDNA的形成过程,但不能清除cccDNA,且具有较多不良反应[6-7]。因此,开发更为有效的慢性乙型肝炎治疗药物是迫切而必要的[8]。一系列针对病毒生活史的关键步骤以及免疫相关宿主因子的候选治疗药物正处于研发阶段,本文将对其进行综述和讨论。
1 针对病毒复制周期HBV属于嗜肝DNA病毒科,其基因组DNA是部分双链的半开环DNA (relaxed circular DNA,rcDNA),全长约为3.2 kb。HBV进入人体后,成熟的HBV病毒颗粒通过表面大蛋白的preS1与肝细胞表面受体-钠离子-牛磺胆酸协同转运多肽(sodium taurocholate cotransporting polypeptide,NTCP)结合进入肝细胞质中[9]。HBV在细胞质中脱去核衣壳,释放出rcDNA,并进入肝细胞核内形成超螺旋结构的cccDNA。在宿主RNA聚合酶Ⅱ的作用下,以cccDNA为模板转录出4种mRNA,长度分别为3.5、2.4、2.1、0.7 kb,其中3.5 kb的mRNA含有HBV DNA序列上全部遗传信息,称为前基因组RNA (pgRNA)。pgRNA进入肝细胞质,与HBV聚合酶结合,被核心蛋白包裹形成核衣壳。然后以pgRNA为模板,在HBV聚合酶的作用下,合成负链DNA,同时pgRNA被降解;再以负链DNA为模板,合成正链DNA,形成子代的rcDNA,包裹了rcDNA的核衣壳在内质网中进行包膜化形成成熟的HBV颗粒分泌到肝细胞外。胞质中的子代rcDNA也可进入肝细胞核内,回补cccDNA池(图 1)。

|
| 图 1 乙型肝炎病毒生命周期以及主要正在研发的抗病毒药物 Figure 1 HBV life cycle and main classes of antivirals in development |
1.1 抑制病毒入侵
HBV通过病毒包膜蛋白与肝细胞表面受体结合侵入肝细胞。阻断包膜蛋白与肝表面受体结合可以抑制病毒再感染并保护尚未被感染的肝细胞[10-11],从而控制疾病的发展。
中和抗体作用于病毒包膜的抗原表位,是最早的HBV感染抑制剂。乙型肝炎病毒免疫球蛋白(HBIG)是作用于HBV表面抗原(hepatitis B surface antigen,HBsAg)的多克隆抗体,能通过阻断表面抗原膜外亲水区与细胞表面的硫酸乙酰肝素蛋白多糖相互作用进而抑制HBV对肝细胞的吸附,已成功应用于乙肝母婴传播的阻断,有研究表明HBIG也能在一定程度上可显著降低小鼠和CHB患者血清中的HBV DNA和HBV表面抗原的水平[12-13]。
另外,一种新型的人源化单克隆抗体(G12)对S蛋白具有较高的特异性和亲和力,能显著降低小鼠血清中HBsAg的水平[14]。最近,Li等报道了一类作用于preS1区域的单克隆抗体,可高效阻断HBV与肝细胞表面特异性受体的结合,从而具有很强的中和活性;这一抗体能在人肝嵌合FRG小鼠模型有效阻断病毒扩散,但是否能有效抑制已经建立的持续性HBV感染,尚待进一步研究[15]。
合成脂肽Myrcludex-B可与HBV-preS1竞争结合NTCP,从而抑制HBV进入肝细胞。在人肝嵌合小鼠模型中,Myrcludex-B不仅能够抑制已感染肝细胞内HBV的传播扩散,而且在感染早期用药可以有效抑制肝细胞内cccDNA水平[16]。该药已经进行临床Ⅱ期评估,数据显示该药具有良好的耐受性和安全性[17]。另外还有一些靶向NTCP的小分子化合物正在研究中,例如Cyclosporin A[18]、Ezetimibe[19]和Irbesartan[10]等。
1.2 靶向共价闭合环状DNA (cccDNA)cccDNA作为病毒复制的模板,主要来源于感染进入的病毒和子代rcDNA的回补[20]。在细胞核内,cccDNA和组蛋白、非组蛋白(核心蛋白和HBx)等结合形成微染色体结构稳定存在,难以被清除,这导致了HBV的持续感染、耐药以及抗病毒治疗停药后HBV再激活[20]。因此,cccDNA的清除有望彻底治愈CHB。目前针对cccDNA的策略包括抑制其形成、抑制其转录以及诱导降解。
两种双取代磺胺类药物CCC-0975和CCC-03436被证实具有干扰rcDNA向cccDNA转化的作用,但不影响已形成的cccDNA[21]。因此,无法彻底清除cccDNA。
近年来,许多研究表明cccDNA的转录活性受“组蛋白编码”支配[22],主要是组蛋白的修饰(甲基化、乙酰化或者泛素化等)[23]。有研究表明,在细胞和人源化小鼠肝细胞中,给予IFNα处理可引起cccDNA结合的组蛋白低乙酰化并招募转录抑制因子到cccDNA上,同时减少STAT1/2转录因子与cccDNA的结合[24]。此外,许多研究发现非组蛋白HBX能够通过与E3泛素连接酶复合物上的DNA损伤结合蛋白(DDB1)结合降解相关转录调节蛋白,从而达到抑制cccDNA转录功能[20, 25]。由于抑制cccDNA的转录功能只是短暂的效果,这种策略难以实现彻底治愈的目标。
如何降解已形成的cccDNA是研究的重点。研究报道,在分化的HepRG和原代肝细胞模型中发现干扰素α (IFNα)和淋巴毒素β受体(LTβR)激动剂能分别上调两种胞嘧啶脱氨酶APOBEC3A和APOBEC3B,然后通过乙型肝炎病毒核心蛋白介导胞嘧啶脱氨酶结合到cccDNA上,使其胞嘧啶脱氨,并最终被特异性降解[26]。这一理论在一定程度上提供了非细胞毒性方式清除cccDNA的新机制,但结果的可靠性仍有待进一步研究[27]。与此相似,研究者发现T细胞来源的干扰素γ和肿瘤坏死因子同样可以依赖胞嘧啶脱氨酶实现对cccDNA的非细胞毒性降解[28]。此外,许多研究者利用基因治疗的方法将cccDNA特异性核酸酶或者特定序列核酸内切酶运送至感染细胞中降解cccDNA[1, 29],包括CRISPR/Cas9系统[29-31]、锌指核酸酶(ZFNs)[32-33]、大范围核酸酶(meganuclease)、类转录激活核酸酶(TALENs)[34-35]等。然而,目前基因编辑技术存在潜在的脱靶效应和缺乏高效且特异靶向的载体等局限,仍有待进一步研究[36-38]。
1.3 靶向病毒mRNA反义寡核苷酸、核酶和RNA干扰(RNAi)都可以靶向HBV mRNA。反义寡核苷酸主要依赖RNase H通路,目前有两种药进入临床试验,如IONIS-HBVRX/IONIS-HBV-LRX[39]。而RNAi是指由双链小RNA [包括小干扰RNA(siRNA)和微小RNA (microRNA)]诱发的同源mRNA高效特异性降解的现象[40]。由于siRNA沉默病毒基因的特异性、高效性以及近年来药物投递材料的改进,使其成为研究的新热点。目前,有5种siRNA分子正在临床试验中,包括ARB-1467/ARB-1740 (引自http://investor.arbutusbio.com/releasedetail.cfm?ReleaseID=1022375)、ALN-HBV、RG6004\ARO-HBV (表 1)。
| Classification | Drug name | Stage of development | ClinicalTrials.gov identifier | Company |
| Entry inhibitors | Myrcludex-B | Ⅱb | NCT02888106 | Hepatera Ltd |
| Antisense molecules | IONIS-HBVRX/IONIS-HBV-LRX | ⅡⅡ | NCT02981602/ NCT03020745 | GSK |
| siRNAs | ARB-1467 | Ⅱb | NCT02631096 | Arbutus Biopharma |
| siRNAs | ARB-1740 | Ⅱ | No identifier found | Arbutus Biopharma |
| siRNAs | ALN-HBV | Ⅰ/Ⅱ | NCT02826018 | Alnylam Pharmaceuticals |
| siRNAs | RG6004 (HBV LNA) | Ⅰ/Ⅱ | No identifier found | Roche |
| siRNAs | ARO-HBV | Ⅰ/Ⅱ | NCT03365947 | Arrowhead Pharmaceuticals |
| siRNAs | Hepbarna (BB-HB-331) | Preclinical | No identifier found | Benitec |
| siRNAs | Lunar-HBV | Preclinical | No identifier found | Arcturus |
| TDF Pro drugs | TXL (CMX-157) | Ⅱ | NCT02710604 | ContraVir Pharmaceuticals |
| Capsid inhibitors | NVR 3-778 | Ⅱ | NCT03125213 | Janssen |
| Capsid inhibitors | Morphothiadin (GLS-4) | Ⅱ | NCT03638076 | HEC Pharma, PR China |
| Capsid inhibitors | AIC 649 | Ⅰ | No identifier found | AiCuris |
| Capsid inhibitors | JNJ56136379 | Ⅰ | No identifier found | Janssen |
| Capsid inhibitors | ABI-H0731 | Ⅱ | NCT03577171/ NCT03576066 | Assembly Biosciences |
| Capsid inhibitors | AB-423 | Ⅰ | No identifier found | Arbutus Biopharma |
| Capsid inhibitors | EP-027367 | Preclinical | No identifier found | Enanta Pharmaceuticals |
| HBsAg inhibitors | REP-2139 | Ⅱ | NCT02646189/ NCT02233075/ NCT02726789/ NCT02565719 | Replicor |
| HBsAg inhibitors | REP-2165 | Ⅱ | NCT02565719 | Replicor |
| Ribonuclease H inhibitors | RNaseH Inhibitor | Preclinical | No identifier found | Arbutus Biopharma |
| Remarks: Data from Hepatitis B Foundation and ClinicalTrials. | ||||
ARC520是Arrowhead公司研发的一种多重共轭抗HBV siRNA药物,能够降解9种基因型HBV转录产物[41]。ARC520可显著降低猩猩体内HBV RNA、DNA和病毒蛋白水平,在HBeAg阳性病人中效果更为显著[42-43]。临床Ⅱ期试验结果表明,ARC520与NAs联用能够明显降低慢乙肝患者血清中HBsAg和HBeAg水平[44]。此外,ARC-520可以提高抗HBV的T细胞反应[45]。ARC-521是第二代siRNA,可同时作用于cccDNA和整合形式的病毒片段DNA转录的mRNA产物,适用于具有HBsAg转录模板片段整合的慢性乙肝患者(数据引自:http://ir.arrowheadpharma.com/releasedetail.cfm?ReleaseID=967823)。不幸的是,由于基于高剂量EX1在非人类灵长类动物中进行的毒理学研究出现了死亡事件,ARC-520正在进行的Ⅱb期临床研究被FDA暂停。
ALN-HBV主要利用纳米颗粒将其运输至肝细胞的siRNA。在AAV-HBV转导的小鼠上连续注射三针,剂量为3 mg/kg,HBsAg水平平均下降了2.9 log10,并且低水平维持了100 d (数据引自:http://www.alnylam.com/web/assets/ALN-HBV_AASLD_111515.pdf)。此外,在猩猩模型实验结果显示,药物注射后第60天HBsAg平均下降了2 log10 (数据引自:http://www.alnylam.com/web/assets/ALN-HBV_RNAi_Roundtable_072815.pdf)。
虽然siRNA抑制HBV效果显著,但仍然存在有待突破的技术难点。例如siRNA进入机体后可能会刺激机体的天然免疫系统,引发炎性细胞分子的分泌或者引起干扰素反应[46]。除此之外,有研究表明siRNA可能会与内源miRNA机制存在竞争关系,需要进一步研究寻找沉默性与毒性之间的平衡点,以小剂量达到最好的抑制效果。即便能够安全定向地将药物运送至靶细胞,siRNA在与靶基因结合时也可能出现脱靶现象。
1.4 新核苷(酸)类似物目前临床一线治疗药物NAs最大的局限性是长期治疗易产生耐药性。为了克服这一缺点,许多优化的NAs正在临床研究中。
替诺福韦艾拉酚胺富马酸(Tenofovir alafenamide fumarate,TAF)是新一代的替诺福韦前体(Tenofovir disoproxil fumarate,TDF)药物,其在血液和组织中更加稳定,抗病毒效能更强,TAF只需要十分之一的TDF给药剂量,即可实现与TDF相同的抗病毒疗效。同时副作用降低,能有效改善骨骼安全性系数,降低骨质疏松症风险,且对于肾脏的危害更小[47]。另外Ⅲ期临床试验均未发现耐药,可实现临床治愈[48]。目前,TAF已经通过FDA认证,有望成为慢性乙型肝炎治疗的一线药。
贝西福韦(Besifovir)是一种与阿德福韦酯结构类似的口服抗HBV药物。为期2年的多中心临床试验表明,采用贝西福韦治疗的患者,其病毒转阴率、转氨酶复常率和HBeAg阴转率与ETV相比无明显差异,且未发生耐药。然而,贝西福韦一项突出的副作用是会导致体内肉碱水平下降,但可以通过额外补充予以纠正。目前,贝西福韦正在进行Ⅲ期临床试验,虽然疗效并未超越现有药物,但为患者提供了更多选择。
1.5 抑制核衣壳组装核衣壳是rcDNA合成的场所,在HBV复制过程中,核衣壳保护病毒基因组免于降解,同时在感染过程中介导病毒基因组释放,这种双重的作用对于病毒的扩增具有重要作用[49]。苯基丙烯酰胺类化合物(如AT-130、AT-61等)通过改变HBV衣壳蛋白的空间结构[50-54],从而阻止RNA进入核衣壳内。甲磺酸莫非赛定(GLS-4)是一种二氢嘧啶类(HAPs)药物,可诱导核衣壳的错误装配,从而抑制HBV的复制及成熟病毒颗粒的产生[55-56],且对NAs耐药病毒株均有明显抑制作用[57-58]。另外,有研究发现HAPs能影响cccDNA的稳定性[59]。NVR3-778作为核心蛋白抑制剂,也能引诱衣壳错误组装。临床Ⅰb试验结果表明,NVR3-778具有良好耐受性,能够降低CHB患者的HBV DNA及早期HBeAg水平,且与PEG-IFN-α联用的效果优于单用[60]。异噻氟定(NZ-4)主要通过靶向HBV核心蛋白C末端的第150–152位精氨酸,诱导HBV病毒颗粒的异常组装、形成不含病毒基因的空载病毒颗粒,从而阻断病毒的正常复制[61-62]。
1.6 抑制乙型肝炎病毒表面抗原(HBsAg)HBsAg的大量存在是导致和维持慢性HBV感染过程中免疫耐受的重要原因,HBsAg阴转被认为是慢乙肝得到控制的最佳预后指标[63]。然而目前的治疗药物和方案较难实现HBsAg阴转。开发更为有效的可清除或显著降低HBsAg的新药有望提高慢性乙肝的临床治愈率。
目前临床研究进展较快的HBsAg抑制剂是一类核酸聚合物(nucleic acid polymer,NAP),具有广谱抗病毒活性,特别是能抑制HBsAg的分泌[64-66]。在鸭模型中发现NAP REP 2055能够快速清除HBsAg,同时抗-HBs水平持续上升,且在肝细胞中检测不到病毒抗原[67]。REP2139-Ca是REP2055的类似物,两者抗病毒能力相当,但在血液中更加稳定,且能显著降低患者血清HBV RNA水平,但是对HBV-RNA的作用机制尚未得知[68-69]。三项概念验证临床试验评估了REP2055单用以及REP2139-Ca联用PEG-IFNa/ETV对慢乙肝患者的耐受性和抗病毒效应[70-71]。结果显示,NAP联合免疫治疗和ETV是安全的。而且与单用REP2055比较,REP2139-Ca联合PEG-IFNa具有协同抗病毒效应,同时增加了持续病毒学应答的比率。进一步,一项随机对照试验(NCT02565719)结果表明:NAPs介导的血清HBsAg清除与转氨酶水平爆发密切相关,且转氨酶水平提升并没有影响肝功能,提示转氨酶的升高是清除被感染肝细胞的自然反应。同时发现NAP介导的HBsAg清除提高了peg-IFNα的治疗效果(数据引自:http://replicor.com/wp-content/uploads/2016/11/Replicor-REP-401-AASLD-2016-LB-7.pdf,http://replicor.com/wp-content/uploads/2017/04/REP-401-EASL-2017-FINAL-THU-154.pdf)。
最近,我们团队研究出一种针对HBsAg单克隆抗体(E6F6),给予HBV转基因小鼠单剂量的E6F6表现出更强效、更持久的抑制HBsAg水平和HBV DNA水平,且抑制持续时间长达数周之久。E6F6抗体主要具有两方面的治疗作用:其一是抗体介导的中和作用,在基于分化的HepaRG/HBV感染细胞模型和基于FRG小鼠的人肝嵌合小鼠(Hu-FRG)体内感染模型中均证实了E6F6能有效阻断HBV感染肝细胞,即使对已经感染HBV的Hu-FRG小鼠,E6F6治疗仍然能显著抑制肝内感染的扩散;其二是抗体介导的高效病毒清除作用,在高复制的HBV转基因小鼠、基于尾静脉高压注射的HBV携带小鼠(HDI-HBV)和HBV感染的Hu-FRG小鼠中均证实单剂E6F6治疗即可实现对HBsAg/HBV DNA长达20 d以上的持续抑制,并且多剂E6F6治疗后能显著提高HDI-HBV小鼠PBMC中HBV特异性T细胞比例,表明E6F6治疗能部分逆转宿主对HBV的免疫耐受。研究证实E6F6介导的HBV清除作用主要由FcγRs介导吞噬细胞(如Neutrophil、Macrophage等)对病毒及病毒抗原的吞噬作用完成[72]。E6F6的多针次长期用药可显著降低二乙基亚硝胺(diethylnitrosamine,DEN)诱导的HBV导致的小鼠肝癌发生率[73]。
2 免疫调节在感染急性期HBV的清除依赖机体及时诱发广泛、强烈的T细胞反应[45, 74]。然而,在慢性感染过程中,由于乙型肝炎病毒抗原的长期大量分泌、先天免疫细胞激活不足和抗原递呈细胞功能缺失、免疫抑制细胞的比例上调等因素形成免疫耐受环境[45, 74],表现为HBV特异T细胞数量缺失和功能受损。同时,自然杀伤细胞(NK)和树突状细胞(DC)等天然免疫细胞的功能也表现为功能受损状态,最终导致在慢性感染情况下机体缺乏有效对抗病毒的免疫反应[75-76]。
2.1 恢复固有免疫功能作为机体抗病毒感染的第一道防线,固有免疫系统能够快速识别病毒核酸、蛋白等成分,并启动系列抗病毒免疫应答。高效的固有免疫应答是控制病毒复制、决定疾病进展的关键环节。
2.1.1 Toll样受体(Toll-like receptor,TLR)激动剂:TLRs是一类重要的天然免疫应答分子,参与识别多种病原体相关分子模式,启动针对入侵病原体的早期应答,诱发获得性免疫反应,是连接天然免疫系统和获得性免疫系统的桥梁[76]。然而,许多证据表明HBV可调节TLRs的表达或抑制TLR的信号通路,逃避TLR介导的天然免疫应答[77-78]。
GS-9620是一种口服的TLR7激动剂,具有较强的抗病毒效应,其在黑猩猩HBV感染模型中的应用可以实现病毒的长期抑制并促进IFN-α的产生[79]。进一步在土拨鼠上验证,结果显示GS-9620能够长期抑制血液和肝脏的HBV DNA,同时对cccDNA也有作用[80]。此外,一项随机对照、双盲的临床研究表明,GS-9620是相对安全和耐受的,接受GS-9620治疗CHB患者并未观察到HBsAg和HBV DNA的显著下降,其外周血的干扰素刺激基因15(ISG15)表达上调,且呈剂量关系[81]。目前该药正在进行临床Ⅱ期试验。
RG-7795是第二个口服TLR7激动剂,最开始研发用于治疗HCV[82]。目前已被罗氏公司用于抗HBV的研究,进入临床Ⅱ期。
2.2 恢复适应性免疫功能适应性免疫应答,尤其是T细胞介导的细胞免疫应答对于控制HBV病毒复制与慢乙肝的进展至关重要。
2.2.1 治疗性疫苗:在慢乙肝感染中,有效的抗体和T细胞反应对于病毒的清除至关重要,但是在慢性感染过程中T细胞耗竭导致病毒无法被清除[83]。治疗性疫苗是一种基于特异性主动免疫的制剂,通过设计HBV特异抗原,刺激CHB患者免疫系统,打破免疫耐受,通过肝细胞杀伤或者非杀伤途径,特异性抑制和清除病毒,达到治疗目的。
目前乙肝治疗性疫苗主要包括蛋白类疫苗、表位肽类疫苗、DNA类疫苗和DCs疫苗等。治疗性疫苗在动物模型上显示出良好的效果,可以通过诱导T淋巴细胞免疫反应限制病毒感染,但是临床试验结果并不理想。有研究认为高病毒载量会导致免疫功能缺陷[84],因此先给予NUCs,有效降低病毒载量或体内抗原负荷, 在此基础上接种治疗性疫苗,可能有利于激活T细胞抵御病原体。然而,在一项Ⅰ/Ⅱ期临床试验显示,DNA疫苗联用NAs并没有降低NAs停药后复发风险以及恢复抗病毒免疫应答[85]。因此,如何提高HBV特异性细胞免疫应答的强度,并延长其持续时间,是治疗性疫苗研发过程中亟待解决的问题。可能治疗性疫苗需要联合适合的佐剂、模式受体激动剂或者检查点抑制剂等才能发挥作用[84, 86]。
目前有多项治疗性疫苗处于临床Ⅰ/Ⅱ期,包括GS-4774[87-88]、TG1050[89]、DV601[90]等(表 2)。
| Classification | Drug name | Mechanism | Stage of development | Company | ClinicalTrials.gov identifier |
| Innate immune defense pathway | GS9620 | TLR-7 agonist | Ⅱ | Gilead | NCT02166047/ NCT02579382 |
| Innate immune defense pathway | RO6864018 (RG7795 ANA773) | TLR-7 agonist | Ⅱ | Roche | NCT02391805 |
| Innate immune defense pathway | GS9688 | TLR-8 agonist | Ⅱ | Gilead | NCT03491553/ NCT03615066 |
| Innate immune defense pathway | Inarigivir (SB9200) | RIG-1 and NOD2 agonist | Ⅱ | Spring Bank Pharmaceuticals | NCT02751996/ NCT03434353 |
| Innate immune defense pathway | ABX-203 | NK receptor agonist | Ⅲ | Abivax | NCT02249988 |
| Therapeutic vaccine | TG-1050 | Vaccine technology used to stimulate the immune system as a treatment | Ⅰ/Ⅰb | Transgene | NCT02428400 |
| Therapeutic vaccine | DV-601 | Ⅰb | Dynavax | NCT01023230 | |
| Therapeutic vaccine | HB-110 | Ⅱ | Genexine | NCT01813487 | |
| Therapeutic vaccine | GS-4774 | Ⅱ | Gilead | NCT01943799/ NCT02174276 | |
| Therapeutic vaccine | HepTcell | Ⅰ | Altimmune | NCT02496897 | |
| Therapeutic vaccine | INO-1800 | Ⅰ | Inovio Pharmaceuticals | NCT02431312 | |
| Therapeutic vaccine | TomegaVax HBV | Preclinical | TomegaVax | No identifier found | |
| Therapeutic vaccine | MVA-VLP | Preclinical | GeoVax Labs | No identifier found | |
| Host acting pathway | EYP001 | FXR agonist | Ⅰ | Enyo Pharma | NCT03272009 |
| Host acting pathway | APG-1387 | Apotosis inducer | Ⅰ | Ascentage Pharma, China | NCT03585322 |
| Host acting pathway | CRV 431 (CPI 431-32) | Ciclofillin inhibitor | Ⅰ | ContraVir | NCT03596697 |
| Gene editing | EBT106 | CRISPR/Cas | Preclinical | Excision Biotherapeutics | No identifier found |
| Gene editing | HBV | Preclinical | Intellia Therapeutics | No identifier found | |
| Other | GC1102 | HBsAg monoclonal antibody | Ⅱ | Green Cross | NCT03519113 |
| Other | LTCR-H2-1 | T cell immunotherapy | Preclinical | Lion TCR | No identifier found |
| Remarks: Data from Hepatitis B Foundation and ClinicalTrials. | |||||
2.2.2 免疫检查点抑制剂治疗:
在CHB感染过程中,HBV特异CD8+ T细胞表现为PD-1、CTLA-4、Lag-3、Tim-3、CD244等[45, 91]免疫检验点分子的高表达。免疫检验点疗法即通过靶向阻断共抑制受体的负调控信号达到恢复HBV特异性CD8+ T细胞的功能。
针对免疫检验点的抗体已经在癌症治疗中表型出突破性的疗效[92-93],全球已上市2个PD-1产品和3个PD-L1产品[94-95]。在HBV持续感染的小鼠模型中发现,注射抗PD-1单克隆抗体可逆转了肝内T淋巴细胞的耗竭表型,同时在一定程度上清除HBV[96]。在土拨鼠病毒性肝炎模型中发现,阻断PD-1/PD-L1通路,进一步联合ETV和DNA疫苗,明显增强病毒特异性T细胞的功能以及对病毒复制实现持续的免疫控制[97]。另外,从CHB患者中分离出CD8+ T细胞比较阻断不同检查点抑制受体的效应,包括PD-1、2B4、CLTA-4、Tim-3和BTLA,结果显示,抗PD-1分子恢复免疫功能效果最强[98]。目前已有临床试验探索PD-1抗体在慢性乙肝治疗中的潜力,尚无公开文献数据。
2.2.3 HBV特异性T细胞:另外一种补偿免疫反应耗竭的策略是往CHB患者体内输入体外改造后的自体HBV特异T细胞,主要包括嵌合抗原受体T细胞(CAR-T)和过表达HBV特异性T细胞受体的T细胞(TCR-T)[84]。TCR-T细胞具有有效的识别抗原的能力,但是它存在一定的缺点限制了它的应用,例如HLA限制性以及TCR的低亲和力。CAR-T可以在一定程度上克服以上缺点。首款用于治疗白血病的CAR-T疗法有望在今年上市,这将给CAR-T领域带来新希望。尽管CAR-T疗法在白血病治疗中取得较好的效果,但是其安全性问题仍是关注重点,一方面,细胞因子释放综合征(CRS),另一方面,过表达CAR或者TCR需要逆转录病毒或者慢病毒载体介导,可能导致复制型逆转录病毒以及插入诱变的遗传毒性[99]。
3 展望目前学界已经对乙型肝炎病毒的生命周期有了更为深入的理解,大量靶向HBV生命周期各个点的新型抗病毒药物正在进行或即将进入临床试验,包括进入抑制剂、病毒转录抑制剂、病毒聚合酶抑制剂、核衣壳组装调节剂和HBsAg分泌抑制剂。然而,现阶段仍然难以判断采用何种策略或机制的药物有望真正实现慢性乙肝功能性治愈的大幅提升。在这些正在开发的新药中,TAF等新型NAs类药物只是减少了核苷酸类似物在肾及骨骼方面的不良反应,其Anti-HBV作用与TDF并无显著差异。Myrcludex-B等入胞抑制剂可以有效抑制HBV的传播和再感染,但可能无法清除已存在的HBV。降解cccDNA被认为是最有可能清除HBV的治疗策略,但目前对cccDNA形成、维持及降解的生物学机制尚不完全清楚。特异靶向cccDNA的小分子药物可能成为未来HBV新药发展的重要方向。此外,随着对HBV调节免疫应答机制的理解,以及导致抗病毒T淋巴细胞免疫功能障碍过程的明确,以打破HBV免疫耐受促进患者Anti-HBV免疫应答恢复的免疫治疗策略也可能成为新的突破点。借鉴HCV DAA药物成功的经验,多途径、多靶点、多药物联合阻断HBV的生物学合成,结合免疫治疗将可能成为实现慢性HBV感染根治的重要途径。
| [1] | Durantel D, Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. Journal of Hepatology, 2016, 64(S1): S117-S131. |
| [2] | Frenette CT, Gish RG. Editorial: To "be" or not to "be": that is the question. The American Journal of Gastroenterology, 2009, 104(8): 1948-1952. DOI:10.1038/ajg.2009.204 |
| [3] | Moucari R, Lada O, Marcellin P. Chronic hepatitis B: back to the future with HBsAg. Expert Review of Anti-Infective Therapy, 2009, 7(6): 633-636. DOI:10.1586/eri.09.57 |
| [4] | Revill P, Locarnini S. Antiviral strategies to eliminate hepatitis B virus covalently closed circular DNA (cccDNA). Current Opinion in Pharmacology, 2016, 30: 144-150. DOI:10.1016/j.coph.2016.08.015 |
| [5] | Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. The Lancet, 2014, 384(9959): 2053-2063. DOI:10.1016/S0140-6736(14)60220-8 |
| [6] | Gish RG, Given BD, Lai CL, Locarnini SA, Lau JYN, Lewis DL, Schluep T. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Research, 2015, 121: 47-58. DOI:10.1016/j.antiviral.2015.06.008 |
| [7] | Marcellin P, Ahn SH, Ma XL, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, Petersen J, Foster GR, Lou LL, Martins EB, Dinh P, Lin LJ, Corsa A, Charuworn P, Subramanian GM, Reiser H, Reesink HW, Fung S, Strasser SI, Trinh H, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HLY. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology, 2016, 150(1): 134-144.e10. DOI:10.1053/j.gastro.2015.09.043 |
| [8] | Lin CL, Kao JH. Perspectives and control of hepatitis B virus infection in Taiwan. Journal of the Formosan Medical Association, 2015, 114(10): 901-909. DOI:10.1016/j.jfma.2015.06.003 |
| [9] | Yan H, Zhong GC, Xu GW, He WH, Jing ZY, Gao ZC, Huang Y, Qi YH, Peng B, Wang HM, Fu LR, Song M, Chen P, Gao WQ, Ren BJ, Sun YY, Cai T, Feng XF, Sui JH, Li WH. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife, 2012, 1: e00049. DOI:10.7554/eLife.00049 |
| [10] | Verrier ER, Colpitts CC, Sureau C, Baumert TF. Hepatitis B virus receptors and molecular drug targets. Hepatology International, 2016, 10(4): 567-573. DOI:10.1007/s12072-016-9718-5 |
| [11] | Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology, 2014, 147(1): 48-64. DOI:10.1053/j.gastro.2014.04.030 |
| [12] | Tsuge M, Hiraga N, Uchida T, Kan H, Miyaki E, Masaki K, Ono A, Nakahara T, Abe-Chayama H, Zhang YZ, Naswa MG, Kawaoka T, Miki D, Imamura M, Kawakami Y, Aikata H, Ochi H, Hayes NC, Chayama K. Antiviral effects of anti-HBs immunoglobulin and vaccine on HBs antigen seroclearance for chronic hepatitis B infection. Journal of Gastroenterology, 2016, 51(11): 1073-1080. DOI:10.1007/s00535-016-1189-x |
| [13] | Lempp FA, Urban S. Inhibitors of hepatitis B virus attachment and entry. Intervirology, 2014, 57(3/4): 151-157. |
| [14] | Wang W, Sun LN, Li TS, Ma YC, Li JS, Liu Y, Li M, Wang L, Li C, Xie YH, Wen YM, Liang MF, Chen L, Tong SP. A human monoclonal antibody against small envelope protein of hepatitis B virus with potent neutralization effect. mAbs, 2016, 8(3): 468-477. DOI:10.1080/19420862.2015.1134409 |
| [15] | Li D, He WH, Liu XM, Zheng SD, Qi YH, Li HY, Mao FF, Liu J, Sun YY, Pan LJ, Du KX, Ye KQ, Li WH, Sui JH. A potent human neutralizing antibody Fc-dependently reduces established HBV infections. eLife, 2017, 6: e26738. DOI:10.7554/eLife.26738 |
| [16] | Volz T, Allweiss L, ḾBarek MB, Warlich M, Lohse AW, Pollok JM, Alexandrov A, Urban S, Petersen J, Lütgehetmann M, Dandri M. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. Journal of Hepatology, 2013, 58(5): 861-867. DOI:10.1016/j.jhep.2012.12.008 |
| [17] | Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M, Lehr T, Lempp FA, Wedemeyer H, Haag M, Schwab M, Haefeli WE, Blank A, Urban S. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. Journal of Hepatology, 2016, 65(3): 490-498. DOI:10.1016/j.jhep.2016.04.016 |
| [18] | Watashi K, Sluder A, Daito T, Matsunaga S, Ryo A, Nagamori S, Iwamoto M, Nakajima S, Tsukuda S, Borroto-Esoda K, Sugiyama M, Tanaka Y, Kanai Y, Kusuhara H, Mizokami M, Wakita T. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology, 2014, 59(5): 1726-1737. DOI:10.1002/hep.26982 |
| [19] | Lucifora J, Esser K, Protzer U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral Research, 2013, 97(2): 195-197. DOI:10.1016/j.antiviral.2012.12.008 |
| [20] | Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut, 2015, 64(12): 1972-1984. DOI:10.1136/gutjnl-2015-309809 |
| [21] | Cai DW, Mills C, Yu WQ, Yan R, Aldrich CE, Saputelli JR, Mason WS, Xu XD, Guo JT, Block TM, Cuconati A, Guo HT. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrobial Agents and Chemotherapy, 2012, 56(8): 4277-4288. DOI:10.1128/AAC.00473-12 |
| [22] | Pollicino T, Belloni L, Raffa G, Pediconi N, Squadrito G, Raimondo G, Levrero M. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B Virus cccDNA-bound H3 and H4 histones. Gastroenterology, 2006, 130(3): 823-837. DOI:10.1053/j.gastro.2006.01.001 |
| [23] | Tropberger P, Mercier A, Robinson M, Zhong WD, Ganem DE, Holdorf M. Mapping of histone modifications in episomal HBV cccDNA uncovers an unusual chromatin organization amenable to epigenetic manipulation. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(42): E5715-E5724. DOI:10.1073/pnas.1518090112 |
| [24] | Belloni L, Allweiss L, Guerrieri F, Pediconi N, Volz T, Pollicino T, Petersen J, Raimondo G, Dandri M, Levrero M. IFN-α inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. The Journal of Clinical Investigation, 2012, 122(2): 529-537. DOI:10.1172/JCI58847 |
| [25] | Lucifora J, Protzer U. Attacking hepatitis B virus cccDNA-The holy grail to hepatitis B cure. Journal of Hepatology, 2016, 64(S1): S41-S48. |
| [26] | Lucifora J, Xia YC, Reisinger F, Zhang K, Stadler D, Cheng XM, Sprinzl MF, Koppensteiner H, Makowska Z, Volz T, Remouchamps C, Chou WM, Thasler WE, Hüser N, Durantel D, Liang TJ, Münk C, Heim MH, Browning JL, Dejardin E, Dandri M, Schindler M, Heikenwalder M, Protzer U. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science, 2014, 343(6176): 1221-1228. DOI:10.1126/science.1243462 |
| [27] | Chisari FV, Mason WS, Seeger C. Comment on "Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA". Science, 2014, 344(6189): 1237. |
| [28] | Xia YC, Stadler D, Lucifora J, Reisinger F, Webb D, Hösel M, Michler T, Wisskirchen K, Cheng XM, Zhang K, Chou WM, Wettengel JM, Malo A, Bohne F, Hoffmann D, Eyer F, Thimme R, Falk CS, Thasler WE, Heikenwalder M, Protzer U. Interferon-γ and tumor necrosis factor-α produced by T cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology, 2016, 150(1): 194-205. DOI:10.1053/j.gastro.2015.09.026 |
| [29] | Kennedy EM, Kornepati AVR, Cullen BR. Targeting hepatitis B virus cccDNA using CRISPR/Cas9. Antiviral Research, 2015, 123: 188-192. DOI:10.1016/j.antiviral.2015.10.004 |
| [30] | Seeger C, Sohn JA. Targeting hepatitis B virus with CRISPR/Cas9. Molecular Therapy Nucleic Acids, 2014, 3: e216. DOI:10.1038/mtna.2014.68 |
| [31] | Seeger C, Sohn JA. Complete spectrum of CRISPR/Cas9-induced mutations on HBV cccDNA. Molecular Therapy, 2016, 24(7): 1258-1266. DOI:10.1038/mt.2016.94 |
| [32] | Zimmerman KA, Fischer KP, Joyce MA, Tyrrell DLJ. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. Journal of Virology, 2008, 82(16): 8013-8021. DOI:10.1128/JVI.00366-08 |
| [33] | Cradick TJ, Keck K, Bradshaw S, Jamieson AC, McCaffrey AP. Zinc-finger nucleases as a novel therapeutic strategy for targeting hepatitis B virus DNAs. Molecular Therapy, 2010, 18(5): 947-954. DOI:10.1038/mt.2010.20 |
| [34] | Chen JL, Zhang W, Lin JY, Wang F, Wu M, Chen CC, Zheng Y, Peng XH, Li JH, Yuan ZH. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Molecular Therapy, 2014, 22(2): 303-311. DOI:10.1038/mt.2013.212 |
| [35] | Bloom K, Ely A, Mussolino C, Cathomen T, Arbuthnot P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Molecular Therapy, 2013, 21(10): 1889-1897. DOI:10.1038/mt.2013.170 |
| [36] | Urnov F. Genome editing: The domestication of Cas9. Nature, 2016, 529(7587): 468-469. DOI:10.1038/529468a |
| [37] | Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, Madhavan S, Pan XF, Ran FA, Yan WX, Asokan A, Zhang F, Duan DS, Gersbach CA. In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy. Science, 2016, 351(6271): 403-407. DOI:10.1126/science.aad5143 |
| [38] | Schaefer KA, Wu WH, Colgan DF, Tsang SH, Bassuk AG, Mahajan VB. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nature Methods, 2017, 14(6): 547-548. DOI:10.1038/nmeth.4293 |
| [39] | Billioud G, Kruse RL, Carrillo M, Whitten-Bauer C, Gao DC, Kim A, Chen L, McCaleb ML, Crosby JR, Hamatake R, Hong Z, Garaigorta U, Swayze E, Bissig KD, Wieland S. In vivo reduction of hepatitis B virus antigenemia and viremia by antisense oligonucleotides. Journal of Hepatology, 2016, 64(4): 781-789. DOI:10.1016/j.jhep.2015.11.032 |
| [40] | Pan JQ, Tong SM, Kang L, Tang J. New anti-hepatitis B virus drugs under development and evaluation. Current Opinion in Infectious Diseases, 2016, 29(6): 632-638. DOI:10.1097/QCO.0000000000000318 |
| [41] | Gish RG, Yuen MF, Chan HLY, Given BD, Lai CL, Locarnini SA, Lau JYN, Wooddell CI, Schluep T, Lewis DL. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Research, 2015, 121: 97-108. DOI:10.1016/j.antiviral.2015.06.019 |
| [42] | Xu Z, Chavez D, Guerra B, Littlejohn M, Peterson R, Locarnini S, Gish R, Anzalone C, Kanner S, Goetzmann J, Lanford R, Lewis D, Wooddell C. Treatment of chronically HBV-infected chimpanzees with RNA interference therapeutic ARC-520 LED to potent reduction of viral MRNA, DNA and proteins without observed drug resistance. Journal of Hepatology, 2016, 64(S2): S398. |
| [43] | Wooddell CI, Rozema DB, Hossbach M, John M, Hamilton HL, Chu QL, Hegge JO, Klein JJ, Wakefield DH, Oropeza CE, Deckert J, Roehl I, Jahn-Hofmann K, Hadwiger P, Vornlocher HP, McLachlan A, Lewis DL. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Molecular Therapy, 2013, 21(5): 973-985. DOI:10.1038/mt.2013.31 |
| [44] | Yuen MF, Chan HLY, Liu K, Given B, Schluep T, Hamilton J, Lai CL, Locarnini SA, Lau JYN, Ferrari C, Gish RG. Effective inhibition of cccDNA derived mRNA/viral antigens and tolerability with ARC-520. Hepatology International, 2016, 10(Suppl 1): 1. |
| [45] | Ferrari C. HBV and the immune response. Liver International, 2015, 35(S1): 121-128. |
| [46] | Meng ZJ, Lu MJ. RNA interference-induced innate immunity, off-target effect, or immune adjuvant. Frontiers in Immunology, 2017, 8: 331. |
| [47] | Scott LJ, Chan HLY. Tenofovir alafenamide: a review in chronic hepatitis B. Drugs, 2017, 77(9): 1017-1028. DOI:10.1007/s40265-017-0754-9 |
| [48] | Buti M, Gane E, Seto WK, Chan HLY, Chuang WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HLA, Acharya SK, Flaherty JF, Massetto B, Cathcart AL, Kim K, Gaggar A, Subramanian GM, McHutchison JG, Pan CQ, Brunetto M, Izumi N, Marcellin P, GS-US-320-0108 Investigators. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. The Lancet Gastroenterology & Hepatology, 2016, 1(3): 196-206. |
| [49] | Ward H, Tang L, Poonia B, Kottilil S. Treatment of hepatitis B virus: an update. Future Microbiology, 2016, 11(12): 1581-1597. DOI:10.2217/fmb-2016-0128 |
| [50] | Delaney WE, Edwards R, Colledge D, Shaw T, Furman P, Painter G, Locarnini S. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrobial Agents and Chemotherapy, 2002, 46(9): 3057-3060. DOI:10.1128/AAC.46.9.3057-3060.2002 |
| [51] | Billioud G, Pichoud C, Puerstinger G, Neyts J, Zoulim F. The main Hepatitis B virus (HBV) mutants resistant to nucleoside analogs are susceptible in vitro to non-nucleoside inhibitors of HBV replication. Antiviral Research, 2011, 92(2): 271-276. DOI:10.1016/j.antiviral.2011.08.012 |
| [52] | Feld JJ, Colledge D, Sozzi V, Edwards R, Littlejohn M, Locarnini SA. The phenylpropenamide derivative AT-130 blocks HBV replication at the level of viral RNA packaging. Antiviral Research, 2007, 76(2): 168-177. DOI:10.1016/j.antiviral.2007.06.014 |
| [53] | Katen SP, Chirapu SR, Finn MG, Zlotnick A. Trapping of hepatitis B virus capsid assembly intermediates by phenylpropenamide assembly accelerators. ACS Chemical Biology, 2010, 5(12): 1125-1136. DOI:10.1021/cb100275b |
| [54] | Katen SP, Tan ZN, Chirapu SR, Finn MG, Zlotnick A. Assembly-directed antivirals differentially bind quasiequivalent pockets to modify HBV capsid tertiary and quaternary structure. Structure, 2013, 21(8): 1406-1416. DOI:10.1016/j.str.2013.06.013 |
| [55] | Wu GY, Liu B, Zhang YJ, Li J, Arzumanyan A, Clayton MM, Schinazi RF, Wang ZH, Goldmann S, Ren QY, Zhang F, Feitelson MA. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrobial Agents and Chemotherapy, 2013, 57(11): 5344-5354. DOI:10.1128/AAC.01091-13 |
| [56] | Stray SJ, Bourne CR, Punna S, Lewis WG, Finn MG, Zlotnick A. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(23): 8138-8143. DOI:10.1073/pnas.0409732102 |
| [57] | Wang XY, Wei ZM, Wu GY, Wang JH, Zhang YJ, Li J, Zhang HH, Xie XW, Wang X, Wang ZH, Wei L, Wang Y, Chen HS. In vitro inhibition of HBV replication by a novel compound, GLS4, and its efficacy against adefovir-dipivoxil-resistant HBV mutations. Antiviral Therapy, 2012, 17(5): 793-803. DOI:10.3851/IMP2152 |
| [58] | Ren QY, Liu XC, Luo ZH, Li J, Wang CL, Goldmann S, Zhang JC, Zhang YJ. Discovery of hepatitis B virus capsid assembly inhibitors leading to a heteroaryldihydropyrimidine based clinical candidate (GLS4). Bioorganic & Medicinal Chemistry, 2017, 25(3): 1042-1456. |
| [59] | Belloni L, Li L, Palumbo GA, Chirapu SR, Calvo L, Finn MG, Lopatin U, Zlotnick A, Levrero M. HAPs hepatitis B virus (HBV) capsid inhibitors prevent HBc interaction with the viral minichromosome and selected host cell genes to inhibits transcription and affect cccDNA stability. Digestive and Liver Disease, 2014, 46(Suppl 1): e9. |
| [60] | Yuen MF, Kim DJ, Weilert F, Chan HLY, Lalezari JP, Hwang SG, Nguyen T, Liaw S, Brown N, Klumpp K, Flores O, Hartman G, Gane E. LB06-NVR 3-778, a first-in-class HBV core inhibitor, alone and in combination with peg-interferon (PegIFN), in treatment-naive HBeAg-positive patients: early reductions in HBV DNA and HBeAg. Journal of Hepatology, 2016, 64(S2): S210-S211. |
| [61] | Yang L, Wang YJ, Chen HJ, Shi LP, Tong XK, Zhang YM, Wang GF, Wang WL, Feng CL, He PL, Xu YB, Lu MJ, Tang W, Nan FJ, Zuo JP. Effect of a hepatitis B virus inhibitor, NZ-4, on capsid formation. Antiviral Research, 2016, 125: 25-33. DOI:10.1016/j.antiviral.2015.11.004 |
| [62] | Yang L, Shi LP, Chen HJ, Tong XK, Wang GF, Zhang YM, Wang WL, Feng CL, He PL, Zhu FH, Hao YH, Wang BJ, Yang DL, Tang W, Nan FJ, Zuo JP. Isothiafludine, a novel non-nucleoside compound, inhibits hepatitis B virus replication through blocking pregenomic RNA encapsidation. Acta Pharmacologica Sinica, 2014, 35(3): 410-418. DOI:10.1038/aps.2013.175 |
| [63] | Kondo Y, Ninomiya M, Kakazu E, Kimura O, Shimosegawa T. Hepatitis B surface antigen could contribute to the immunopathogenesis of hepatitis B virus infection. ISRN Gastroenterology, 2013, 2013: 935295. |
| [64] | Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers prevent the establishment of duck hepatitis B virus infection in vivo. Antimicrobial Agents and Chemotherapy, 2013, 57(11): 5299-5306. DOI:10.1128/AAC.01005-13 |
| [65] | Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrobial Agents and Chemotherapy, 2013, 57(11): 5291-5298. DOI:10.1128/AAC.01003-13 |
| [66] | Vaillant A. Nucleic acid polymers: Broad spectrum antiviral activity, antiviral mechanisms and optimization for the treatment of hepatitis B and hepatitis D infection. Antiviral Research, 2016, 133: 32-40. DOI:10.1016/j.antiviral.2016.07.004 |
| [67] | Noordeen F, Scougall CA, Grosse A, Qiao Q, Ajilian BB, Reaiche-Miller G, Finnie J, Werner M, Broering R, Schlaak JF, Vaillant A, Jilbert AR. Therapeutic antiviral effect of the nucleic acid polymer REP 2055 against persistent duck hepatitis B virus infection. PLoS One, 2015, 10(11): e0140909. DOI:10.1371/journal.pone.0140909 |
| [68] | Jansen L, Vaillant A, Stelma F, Kootstra NA, Bazinet M, Al-Mahtab M, Reesink HW. O114: Serum HBV-RNA levels decline significantly in chronic hepatitis B patients dosed with the nucleic-acid polymer REP2139-CA. Journal of Hepatology, 2015, 62(Suppl 2): S250. |
| [69] | Bazinet M, Pantea V, Cebotarescu V, Cojuhari L, Jimbei P, Vaillant A. LO2: Significant reduction of HBsAg and HDV RNA by the nucleic acid polymer REP 2139 in caucasian patients with chronic HBV/HDV co-infection. Journal of Hepatology, 2015, 62(Suppl 2): S257-S258. |
| [70] | Al-Mahtab M, Bazinet M, Vaillant A. Effects of nucleic acid polymer therapy alone or in combination with immunotherapy on the establishment of SVR in patients with chronic HBV infection. Journal of Clinical Virology, 2015, 69: 228. |
| [71] | Al-Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One, 2016, 11(6): e0156667. DOI:10.1371/journal.pone.0156667 |
| [72] | Zhang TY, Yuan Q, Zhao JH, Zhang YL, Yuan LZ, Lan Y, Lo YC, Sun CP, Wu CR, Zhang JF, Zhang Y, Cao JL, Guo XR, Liu X, Mo XB, Luo WX, Cheng T, Chen YX, Tao MH, Shih JW, Zhao QJ, Zhang J, Chen PJ, Yuan YA, Xia NS. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut, 2016, 65(4): 658-671. DOI:10.1136/gutjnl-2014-308964 |
| [73] | Kang XZ, Guo XR, Chen BB, Zhang TY, Yuan Q, Chen PJ, Zhang J, Xia NS. The unique antibody suppresses HBV viremia and reduces hepatocarcinogenesis in HBV-transgenic mice. Human Vaccines & Immunotherapeutics, 2018, 14(7): 1779-1781. |
| [74] | Tan A, Koh S, Bertoletti A. Immune response in hepatitis B virus infection. Cold Spring Harbor Perspectives in Medicine, 2015, 5(8): a021428. DOI:10.1101/cshperspect.a021428 |
| [75] | Martinet J, Dufeu-Duchesne T, Bruder Costa J, Larrat S, Marlu A, Leroy V, Plumas J, Aspord C. Altered functions of plasmacytoid dendritic cells and reduced cytolytic activity of natural killer cells in patients with chronic HBV infection. Gastroenterology, 2012, 143(6): 1586-1596.e8. DOI:10.1053/j.gastro.2012.08.046 |
| [76] | Schuch A, Hoh A, Thimme R. The role of natural killer cells and CD8+ T cells in hepatitis B virus infection. Frontiers in Immunology, 2014, 5: 258. |
| [77] | Ma ZY, Zhang EJ, Yang DL, Lu MJ. Contribution of toll-like receptors to the control of hepatitis B virus infection by initiating antiviral innate responses and promoting specific adaptive immune responses. Cellular & Molecular Immunology, 2015, 12(3): 273-282. |
| [78] | Zhang EJ, Lu MJ. Toll-like receptor (TLR)-mediated innate immune responses in the control of hepatitis B virus (HBV) infection. Medical Microbiology and Immunology, 2015, 204(1): 11-20. DOI:10.1007/s00430-014-0370-1 |
| [79] | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. GS-9620, an oral agonist of toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology, 2013, 144(7): 1508-1517.e10. DOI:10.1053/j.gastro.2013.02.003 |
| [80] | Menne S, Tumas DB, Liu KH, Thampi L, AlDeghaither D, Baldwin BH, Bellezza CA, Cote PJ, Zheng J, Halcomb R, Fosdick A, Fletcher SP, Daffis S, Li L, Yue P, Wolfgang GHI, Tennant BC. Sustained efficacy and seroconversion with the toll-like receptor 7 agonist GS-9620 in the woodchuck model of chronic hepatitis B. Journal of Hepatology, 2015, 62(6): 1237-1245. DOI:10.1016/j.jhep.2014.12.026 |
| [81] | Gane EJ, Lim YS, Gordon SC, Visvanathan K, Sicard E, Fedorak RN, Roberts S, Massetto B, Ye ZS, Pflanz S, Garrison KL, Gaggar A, Mani Subramanian G, McHutchison JG, Kottilil S, Freilich B, Coffin CS, Cheng W, Kim YJ. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. Journal of Hepatology, 2015, 63(2): 320-328. DOI:10.1016/j.jhep.2015.02.037 |
| [82] | Bergmann JF, De Bruijne J, Hotho DM, De Knegt RJ, Boonstra A, Weegink CJ, Van Vliet AA, Van De Wetering J, Fletcher SP, Bauman LA, Rahimy M, Appleman JR, Freddo JL, Janssen HLA, Reesink HW. Randomised clinical trial: anti-viral activity of ANA773, an oral inducer of endogenous interferons acting via TLR7, in chronic HCV. Alimentary Pharmacology & Therapeutics, 2011, 34(4): 443-453. |
| [83] | Barnes E. Therapeutic vaccines in HBV: lessons from HCV. Medical Microbiology and Immunology, 2015, 204(1): 79-86. DOI:10.1007/s00430-014-0376-8 |
| [84] | Zhang EJ, Kosinska A, Lu MJ, Yan HM, Roggendorf M. Current status of immunomodulatory therapy in chronic hepatitis B, fifty years after discovery of the virus: Search for the " magic bullet" to kill cccDNA. Antiviral Research, 2015, 123: 193-203. DOI:10.1016/j.antiviral.2015.10.009 |
| [85] | Fontaine H, Kahi S, Chazallon C, Bourgine M, Varaut A, Buffet C, Godon O, Meritet JF, Saïdi Y, Michel ML, Scott-Algara D, Aboulker JP, Pol S, ANRS HB02 Study Group. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: a randomised trial-ANRS HB02 VAC-ADN. Gut, 2015, 64(1): 139-147. DOI:10.1136/gutjnl-2013-305707 |
| [86] | Liang F, Lindgren G, Sandgren KJ, Thompson EA, Francica JR, Seubert A, De Gregorio E, Barnett S, O'Hagan DT, Sullivan NJ, Koup RA, Seder RA, Loré K. Vaccine priming is restricted to draining lymph nodes and controlled by adjuvant-mediated antigen uptake. Science Translational Medicine, 2017, 9(393): eaal2094. DOI:10.1126/scitranslmed.aal2094 |
| [87] | Gaggar A, Coeshott C, Apelian D, Rodell T, Armstrong BR, Shen G, Subramanian GM, McHutchison JG. Safety, tolerability and immunogenicity of GS-4774, a hepatitis B virus-specific therapeutic vaccine, in healthy subjects: a randomized study. Vaccine, 2014, 32(39): 4925-4931. |
| [88] | Lok AS, Pan CQ, Han SHB, Trinh HN, Fessel WJ, Rodell T, Massetto B, Lin LJ, Gaggar A, Subramanian GM, McHutchison JG, Ferrari C, Lee H, Gordon SC, Gane EJ. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. Journal of Hepatology, 2016, 65(3): 509-516. DOI:10.1016/j.jhep.2016.05.016 |
| [89] | Martin P, Dubois C, Jacquier E, Dion S, Mancini-Bourgine M, Godon O, Kratzer R, Lelu-Santolaria K, Evlachev A, Meritet JF, Schlesinger Y, Villeval D, Strub JM, van Dorsselaer A, Marchand JB, Geist M, Brandely R, Findeli A, Boukhebza H, Menguy T, Silvestre N, Michel ML, Inchauspé G. TG1050, an immunotherapeutic to treat chronic hepatitis B, induces robust T cells and exerts an antiviral effect in HBV-persistent mice. Gut, 2015, 64(12): 1961-1971. DOI:10.1136/gutjnl-2014-308041 |
| [90] | Plotkin SA, Schaffner W. A hepatitis B vaccine with a novel adjuvant. Vaccine, 2013, 31(46): 5297-5299. DOI:10.1016/j.vaccine.2013.09.019 |
| [91] | Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death & Disease, 2015, 6: e1694. |
| [92] | Heidelberger V, Goldwasser F, Kramkimel N, Jouinot A, Franck N, Arrondeau J, Guégan S, Mansuet-Lupo A, Alexandre J, Damotte D, Avril MF, Dupin N, Aractingi S. Clinical parameters associated with anti-programmed death-1(PD-1) inhibitors-induced tumor response in melanoma patients. Investigational New Drugs, 2017, 35(6): 842-847. DOI:10.1007/s10637-017-0476-6 |
| [93] | Vafadar S. Immunotherapy for lung cancer: Targeting the PD-1 pathway. Journal of the American Academy of Physician Assistants, 2017, 30(6): 48-50. DOI:10.1097/01.JAA.0000515557.14603.c7 |
| [94] | Three drugs approved for urothelial carcinoma by FDA. Cancer Discovery, 2017, 7(7): 659-660. |
| [95] | Ellis PM, Vella ET, Ung YC. Immune checkpoint inhibitors for patients with advanced non-small-cell lung cancer: a systematic review. Clinical Lung Cancer, 2017, 18(5): 444-459.e1. DOI:10.1016/j.cllc.2017.02.001 |
| [96] | Tzeng HT, Tsai HF, Liao HJ, Lin YJ, Chen LP, Chen PJ, Hsu PN. PD-1 blockage reverses immune dysfunction and hepatitis B viral persistence in a mouse animal model. PLoS One, 2012, 7(6): e39179. DOI:10.1371/journal.pone.0039179 |
| [97] | Liu J, Zhang EJ, Ma ZY, Wu WM, Kosinska A, Zhang XY, Möller I, Seiz P, Glebe D, Wang BJ, Yang DL, Lu MJ, Roggendorf M. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathogens, 2014, 10(1): e1003856. DOI:10.1371/journal.ppat.1003856 |
| [98] | Bengsch B, Martin B, Thimme R. Restoration of HBV-specific CD8+ T cell function by PD-1 blockade in inactive carrier patients is linked to T cell differentiation. Journal of Hepatology, 2014, 61(6): 1212-1219. DOI:10.1016/j.jhep.2014.07.005 |
| [99] | Li KY, Lan YL, Wang JB, Liu LX. Chimeric antigen receptor-engineered T cells for liver cancers, progress and obstacles. Tumour Biology, 2017, 39(3): 1010428317692229. |
 2019, Vol. 59
2019, Vol. 59