中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- Xin Zhai, Xin Yan, Qingwen Meng, Wenting Li, Jintian He, Gaizhen Wang. 2021
- 翟新, 颜鑫, 孟庆文, 李文婷, 贺进田, 王改珍. 2021
- Adjuvant effects of chimeric flagellin and chitosan/tripolyphosphate (CS/TPP) nanogel encapsulation on immune response induced by nasal vaccination of Helicobacter pylori adhesin
- 嵌合鞭毛蛋白和壳聚糖/三聚磷酸(CS/TPP)纳米凝胶封装对鼻接种幽门螺杆菌黏附素诱导的免疫应答的佐剂作用
- Acta Microbiologica Sinica, 61(11): 3569-3582
- 微生物学报, 61(11): 3569-3582
-
文章历史
- 收稿日期:2021-02-03
- 修回日期:2021-04-13
- 网络出版日期:2021-07-08
2. College of Environmental Science and Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, Hebei Province, China
2. 河北科技大学环境科学与工程学院, 河北 石家庄 050018
Helicobacter pylori is a human pathogen that has successfully infected more than half of global population, causing chronic gastritis, gastric and duodenal ulcers, and gastric cancer[1]. The immune response induced by H. pylori cannot clear H. pylori. The current antimicrobial therapy for H. pylori infection includes triple or quadruple antibiotic therapy in combination with one proton pump inhibitor (PPI), which causes serious side effects and potential reinfections[2]. Since most of H. pylori infection occured in childhood, such treatment needs to be used with caution[3]. Therefore, vaccination might be a better strategy to treat with H. pylori infection.
A variety of H. pylori (HP) components have been investigated to prepare vaccines for prevention of HP infections. These components include urease, H. pylori adhesin A (HpaA), flagellin, vacuolating cytotoxin A, neutrophil activation protein, heat shock protein, etc[4]. Among these antigens, HpaA is a lipoprotein on the surface of H. pylori which has been recognized as an essential colonization factor for HP in mice[5]. All HP strains have HpaA genes, and amino acid sequences of HpaA gene are highly conserved. HpaA can induce antigen presentation by human dendritic cells[6]. HpaA is considered as a candidate antigen for H. pylori vaccine. Oral full length HpaA or truncated protein of HpaA and urease could induce effective immune responses against H. pylori infection in mice[7-8]. However, cholera toxin was used as an intramolecular adjuvant in oral vaccination of HpaA, which has inherent toxicity and prevent its use in humans[9]. Investigation on other adjuvants and routes of immunization would be meaningful for development of more effective vaccines against H. pylori infection.
Needle-free mucosal immunization through nasal cavity is an interesting route that has been explored over many years[10]. Nasal immunization possesses many advantages for vaccine delivery than other routes of delivery, such as avoiding first-pass metabolism by the liver, enzymatic degradation in the gastrointestinal tract, slow absorption and low bioavailability[11]. Moreover, there is follicle-associated epithelium overlaying the nasopharynx-associated lymphoid tissue that facilitate inducing strong systemic and local immune responses. Nasal vaccination could effectively induce not only humoral immune responses but also mucosal immune responses in the gastric and the respiratory mucosal[10]. Thus, nasal vaccination might be an ideal vaccination method for prevention of HP infections.
The flagella of HP plays an important role in HP colonization, immune inflammation and immune escape[1]. HP Flagellin FlaA has four structural domains which is D0, D1, D2 and D3 domain, respectively. D0/D1 domains form the core of flagellin, consisted of a highly conserved N- and C-terminal sequence. D2/D3 domains form outer surface of flagellin, consisted of highly variable central region of flagellin. Flagellin is the only known ligand for Toll-like receptor 5. It is known that flagellin activates the natural immune response through at least three signaling pathways[12]. Flagellin can bind with TLR5 through the D1 domain and activates NF-κB, leading to the release of pro-inflammatory cytokines, such as interleukin-6. Flagellin can also bind to NAIP5 (NOD-like receptor family, apoptosis inhibitory protein 5) and NLRC4 (NOD-like receptor containing caspase-associated recruitment domain 4), leading to activation of NLRC4 inflammasome-1[13]. Therefore, flagellin has the potential to activate innate and adaptive immune response through multiple signal pathways, and then generate Th1-Th2 response, humoral and cell-mediated immune response[12-13]. Flagellin has been widely explored as an adjuvant for development of many vaccines and especially Salmonella typhimurium FliC (STF1) or fljB (STF2) flagellin was mostly investigated either co-administered with antigen, or fused with antigen[14]. Various flagellin-antigen fusion vaccines have successfully entered clinical phase 1 trials[14]. However, mutation at D1 domain of the natural HP flagellin led to weak binding ability of HP flagellin to TLR5, which led to evasion of HP from TLR5 recognition[1].
In the present study, chimeric flagellin cFLN was constructed by combining D0, D1 domains of Salmonella typhimurium flagellin FliC with D2, D3 domains of H. pylori flagellin FlaA. It was expected that the chimeric flagellin could restore the binding ability to TLR5, possess an intramolecular adjuvant and antigenic properties[15]. cFLN was further linked to adhesin (HpaA) to construct a new complex antigen cFLN-HpaA. Moreover, cFLN-HpaA was encapsulated into chitosan-based nanogel, it was expected that both cFLN and nanohydrogel encapsulation could enhance together humoral and gastric mucosal immune response induced by nasally delivered HpaA.
1 Materials and Methods 1.1 Materials and animalsIsopropyl-beta-D-thiogalactopyranoside (IPTG), Ni-NTA Prepacked chromatographic column, BCA Protein Assay Kit, Tripolyphosphate (TPP) were purchased from Sangon Biotech Shanghai Co.ltd. (Shanghai, China). Casamino acids and low molecular weight chitosan were purchased from Sigma-Aldrich (St. Louis, MO, USA). DNA polymerase and DNA endonucleases were purchased from TaKaRa Bio (Dalian, China). Fetal bovine serum was purchased from Hangzhou Sijiqing Biology Company (Hangzhou, China). The S. typhimurium (ATCC ID 14028) was obtained from China General Microbiological Culture Collection Center. The ELISA kit was purchased from Shanghai Qiaodu Biological Technology Co. Ltd. (Shanghai, China). Peritoneal macrophages were extracted from female Sprague Dawley rats as described previously[10]. All other chemicals used were analytical grade or better.
The female BALB/c mice (clean animal, 6–7 weeks old, the average weight is 18.12 g) were obtained from Experimental Animal Center of Hebei Medical University. All animal studies were performed at Hebei Normal University and were approved by the Animal Ethics Committee of Hebei Normal University. These mice were treated in accordance with the Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation.
1.2 Construction of expression plasmidsReplacing nucleic acid sequences 385–1235 of D2, D3 domains of S. typhimurium FliC (GenBank ID AY353402.1), nucleic acid sequences 531–1227 of D2, D3 domains in HP flagellin (FlaA, GenBank ID CP003904.1) was joined to nucleic acid sequences 1–384 of D0, D1 domains in amino terminus of S. typhimurium FliC (GenBank ID AY353402.1) and nucleic acid sequences 1236–1512 of D0, D1 domains in carboxyl terminus of S. typhimurium FliC (GenBank ID AY353402.1) to construct chimeric flagellin cFLN (Figure 1). Nucleic acid sequences of HpaA (GenBank ID HQ343312.1) and cFLN was linked with a nucleic acid sequence (GGTTCC GGCGGTTCTGGT, amino acid sequence: GSGGSG) to construct chimeric antigen cFLN-HpaA (Figure 1). Gene sequences of HpaA, cFLN and cFLN-HpaA was synthesized and inserted into plasmid pET-22b to form expression plamids pET-22b-HpaA, pET-22b-cFLN, pET-22b-cFLN- HpaA by Sangon Biotech. Shanghai Co. ltd. (Shanghai, China). The recombinant plasmids were transformed into host bacteria E. coil strain BL21 for antigen expression.

|
| Figure 1 Schematic of construction and design of cFLN and cFLN-HpaA. FliC: Salmonella typhimurium flagellin (blue). FlaA: Helicobacter pylori flagellin (yellow). HpaA: Helicobacter pylori adhesin (HpaA) (pink). |
Amplify cFLN and HpaA used the primers in Table 1.
| Target genes | Primer sequences (5′→3′) | Endonucleases |
| cFLN | GACACCATATGGCGCAGGTTATTAACACCAACAGCCT | Nde Ⅰ |
| CCCTCGAGACGCAGCAGGCTCAGAACG | Xho Ⅰ | |
| HpaA | TTCATATGCGTGCGAACAACCACTTTAAAGACTTCG | Hind Ⅲ |
| GTGTCCTCGAGTTAGTGGTGGTGGTGGTGATG | BamH Ⅰ |
1.3 Expression, refolding and purification of HpaA, cFLN and cFLN-HpaA
Recombinant bacteria were cultured in sterile LB (Luria-Bertani)/ampicillin culture medium. When the OD600 reaches about 1.0, 0.6 mmol/L IPTG was added to induce protein expression. After an additional 4 h of cultivation at 36 ℃, the cells were collected by centrifugation and were then frozen at –40 ℃ or processed immediately.
The collected cells were resuspended in lysis buffer (10 mmol/L phosphate buffered saline, 0.5 mmol/L EDTA, 1 mmol/L Phenylmethanesulfonyl fluoride) and disrupted at 4 ℃ by an 92-IIN ultrasonic cell crusher (Xinzhi Biotechnology Co., Ltd, Ningbo, China). The precipitates were collected by centrifugation. SDS-PAGE analysis indicated that HpaA, cFLN and cFLN-HpaA were expressed as inclusion bodies. Then inclusion bodies were collected by centrifugation at 4 ℃ and washed three times with washing buffer (10 mmol/L Triton X-100, 0.2 mmol/L EDTA, 0.5 mmol/L β-mercaptoethanol, 10 mmol/L phosphate buffered saline) to remove some impurities with a grinding tube. Two grams of inclusion bodies was dissolved in 30 mL dissolved buffer containing 6 mol/L guanidine-HCl (Gu-HCl), 50 mmol/L phosphate buffer (PB), 0.1 mol/L glycerin, 0.5 or 0.7 mol/L arginine. Insoluble material was removed by centrifugation at 10000×g and 4 ℃ for 30 min. The supernatant was put into a dialysis bag with a molecular weight cut-off of 10000 and dialyzed against 500 mL refolding buffer containing 10 mmol/L phosphate buffered saline, 0.5 mmol/L EDTA, 2 mol/L guanidine hydrochloride and 0.5 or 0.7 mol/L arginine for 30 min to remove guanidine hydrochloride. Then the supernatant continued to be dialyzed in turn against 500 mL refolding buffer containing 10 mmol/L phosphate buffered saline, 0.5 mmol/L EDTA, 1 mol/L guanidine hydrochloride and 0.5 or 0.7 mol/L arginine for 60 min, and 500 mL refolding buffer containing 10 mmol/L phosphate buffered saline, 0.5 mmol/L EDTA and 0.5 or 0.7 mol/L arginine overnight. Then solution in the dialysis bag is centrifuged at 10000×g for 15 min at 4 ℃. The supernatant and precipitation were analyzed by SDS-PAGE analysis. The refolding efficiency of protein was determined by percentage of refolding protein to total protein dissolved from inclusion bodies. The protein concentration was determined by BCA Protein Assay Kit according to the manufacturer's instructions.
As expression product has C-terminal tag consisting of six histidine (His) residues, refolded protein could be purified by Ni-NTA Prepacked chromatographic column according to the manufacturer's instructions. The purified antigens were analyzed by SDS-PAGE analysis. Endotoxin within protein products was removed by a EtEraser Endotoxin Removal Kit according to the manufacturer's instructions (Xiamen Bioendo Technology Co., Ltd) before usage.
1.4 Purification of flagellin from Salmonella TyphimuriumFlagellin was purified from Salmonella Typhimurium with a method described previously[16]. The Salmonella typhimurium was inoculated into 300 mL of Luria-Bertani medium and incubated at 37 ℃ overnight. Then the cells were collected by centrifugation at 5000×g at 4 ℃ for 10 min. Physiological saline was used to resuspend the cells. After the cells were washed three times, pH of suspension was adjusted to 2.0 and mildly stirred for 30 min. Then centrifugation at 5000×g for 30 min to remove precipitation. Subsequently, 2.7 mol/L ammonium sulphate was added to precipitate protein overnight at 4 ℃. The protein precipitation was collected by centrifugation at 4 ℃, 15000×g, for 30 min. The resuspended protein was dialyzed in a dialysis bag with a molecular weight cut-off at 50 kDa against 500 mL of 20 mmol/L phosphate-buffered saline overnight. After centrifugation, supernatant containing flagellin was freeze-dried underultra vacuum and stored at –80 ℃. Purified Salmonella Typhimurium flagellin FliC showed a single band in SDS-PAGE analysis (Data not shown). Endotoxin within flagellin was removed by a EtEraser Endotoxin Removal Kit according to the manufacturer's instructions before usage (Xiamen Bioendo Technology Co., Ltd).
1.5 Release of interleukin 6 from macrophages stimulated by flagellin, cFLN and cFLN-HpaARat peritoneal macrophage cells (5×105 cells/well) were seeded at 24-well plate and cultured in the medium containing 88% Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum, 1% double antibody and 1% Non-essential amino acid in an incubator (37 ℃, 5% CO2) overnight. Then culture medium was replaced with DMEM supplemented with 10% fetal calf serum and different concentrations of flagellin, cFLN and cFLN-HpaA ranging between 0.5–8.0 μg/mL. After 12 h of co-culture, cytokine IL-6 in culture supernatant was measured using ELISA kit according to the manufacturer's instructions.
1.6 Preparation of antigen-loaded nanogelcFLN, HpaA and cFLN-HpaA was encapsulated into chitosan nanogels (CS NPs) by ionic gelation method with Tripolyphosphate TPP as a gelating agent[17]. Three percent of chitosan was prepared by dissolving chitosan in 1% (V/V) acetic acid solution. Then pH of chitosan solution was adjusted to 6.0 with NaOH. Different concentrations of TPP were prepared by dissolving TPP in distilled water. 50 μg/mL of cFLN, HpaA and cFLN-HpaA was added into TPP solution. Before usage, chitosan and TPP solution need filter through 0.22 micrometer filter. Then, 40 mL of chitosan solution was mixed with 10 mL of TPP solution containing antigen and the mixture solution was magnetically stirred at ice bath at 600 r/min for one hour. Antigen-loaded chitosan-TPP nanogels formed spontaneously via ionic gelation mechanism. Nanogels were collected by centrifugation at 20000×g for 30 min and were then freeze-dried. Antigen concentrations in the supernatant were measured by BCA Protein Assay Kit to determine the entrapment efficiency and antigen loading.
1.7 Characterization of antigen-loaded nanogel 1.7.1 Determination of entrapment efficiency and antigen loading:Entrapment efficiency (EE) of antigens in nanogels was determined by the following equation.
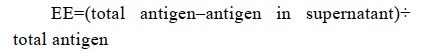
|
公式(1) |
The drug loading (DL) was calculated from the amount of antigen within the NGs to the weight of NGs:

|
公式(2) |
Particle size of antigen-loaded Nanogels was determined with a ZetaSizer nano ZS apparatus (Malvern Instruments Ltd., Malvern, UK).
1.8 Immunization and immune response analysisFemale BALB/c mice (6–7 weeks) were selected, and adequate water and ordinary rat food were given daily at room temperature of about 25 ℃. mice were divided into 7 groups on average, and 5 mice in each group were intranasally inoculated with 20 μg of cFLN, HpaA, cFLN-HpaA solution or cFLN, HpaA, cFLN-HpaA loaded nanogels containing 20 μg of antigen, respectively. The volume of solution inoculated per mice is 10 μL. One group was given PBS as a blank control. Each group of mice was immunized on the 0th, 7th, 14th and 21st days, respectively. Two weeks after the last immunization, the blood samples were collected after eyeball removal. After centrifuged at 3500×g for 10 min, the sera were collected in a EP tube and stored at −20 ℃ until tested.
Before measurement, the serum was diluted 5 times with a 10 mmol/L PBS containing 350 μg/mL phenylmethylsulfonyl, and then IgG, IgG1, IgG2a, IgA concentrations in the serum were measured with IgG, IgG1, IgG2a, IgA ELISA kit according to the manufacturer's instructions, respectively.
Cytokines in stomach of mice immunized with antigen was determined with a method described previously[18]. Briefly, after collecting the blood samples, the mouse was quickly dissected, stomach was took out and gently washed with physiological saline. Then the stomach was put into a mortar, and liquid nitrogen was quickly added into the mortar, subsequently the stomach was grinded into powder. The powder was weighed and resuspended with 500 μL of 10 mmol/L PBS containing 350 μg/mL phenylmethylsulfonyl, 100 μg/mL soybean trypsin inhibitor and 0.1% bovine serum albumin overnight at 4 ℃. then the suspension was centrifuged at 4 ℃ and 9000×g for 10 min. The supernatants were used to determine the production of cytokines IFN-γ, IL-4, IL-17 and SIgA antibodies with IFN-γ, IL-4, IL-17 and SIgA kit according to the manufacturer's instructions, respectively.
1.9 Statistical analysisIn this paper, SPSS 26 is used for data analysis, and the data are expressed as mean±standard deviation (SD). A one-way ANOVA test was used to compare the differences between the groups, where * P < 0.05, ** P < 0.01, *** P < 0.001, indicating a significant difference.
2 Results and Discussion 2.1 Construction, expression, renaturation and purification of cFLN, HpaA and cFLN-HpaATo enhance binding ability of cFLN to TLR 5, D0 and D1 domain of H. pylori flagellin FlaA was replaced with the D0 and D1 domain of S. typhimurium FliC to construct cFLN (Figure 1). Then, C-terminus of cFLN was linked to N-terminus of HpaA with a Gly-Ser flexible linkers to form cFLN-HpaA (Figure 1). Finally, cFLN, HpaA and cFLN-HpaA genes were synthesized and cloned into plasmid pET-22b. The recombinant plasmids were transformed into host bacteria E. coli strain BL21. and expressed in LB medium after induction with IPTG. The soluble and insoluble fractions of E. coli cell lysate were separated by centrifugation and analysed by SDS/PAGE to determine the status of protein expressed in E. coli. SDS-PAGE analysis indicated that HpaA, cFLN and cFLN-HpaA were mainly in the precipitate and expressed as inclusion bodies (Figure 2). As crude inclusion body generally contains many impurities, crude inclusion body of cFLN, HpaA and cFLN-HpaA was firstly washed with 10 mmol/L phosphate buffered saline containing 10 mmol/L Triton X-100[19].

|
| Figure 2 SDS–PAGE analysis of expression products from engineering bacteria and purified proteins. A: cFLN. B: HpaA. C: cFLN-HpaA, lane M: molecular mass standards marker; lane 1: supernatant after engineering bacteria disrupted by sonication; lane 2: precipitation after engineering bacteria disrupted by sonication. D: purified proteins, lane M: molecular mass standards; lane 1: purified cFLN; lane 2: purified HpaA; lane 3: purified cFLN-HpaA. |
Dilution is a commonly used method for refolding proteins from inclusion body[20]. After inclusion body disolved by 6 mol/L guanidine-HCl, renaturation of cFLN, HpaA and cFLN-HpaA was performed with a dialysis-dilution method by dialyzing inclusion body solution against refolding solution containing 0.5 or 0.7 mol/L arginine solution, which was customarily used to promote renaturation of protein[21]. The results indicated that arginine effectively increased the refolding efficiency of cFLN, HpaA and cFLN-HpaA in the concentration range of 0–0.7 mol/L. The refolding efficiency of HpaA and cFLN in 0.7 mol/L Arg could reach 53% and 45% respectively. The refolding efficiency of cFLN-HpaA in 0.5 mol/L Arg could reach 59%. The refolded proteins were subsequently purified by Ni-NTA Prepacked Chromatographic column. Metal ion affinity chromatography purification method efficiently purified cFLN, HpaA and cFLN-HpaA. The purified protein cFLN, HpaA and cFLN-HpaA showed a single band in SDS-PAGE analysis (Figure 2-D).
2.2 Biological activity of chimeric flagellin cFLN and complex antigen cFLN-HpaATo investigate whether the purified protein cFLN and cFLN-HpaA had recovered biological activity, cFLN, FliC and cFLN-HpaA were co-cultured with rat peritoneal macrophage cells. As ligand for Toll-like receptor 5, flagellin can bind with TLR5 and activates NF-κB in macrophage cells, leading to the release of pro-inflammatory cytokines, such as interleukin-6[10]. Thus, interleukin 6 was measured to determine the activation ability of cFLN, and cFLN-HpaA on macrophage cells. The results indicated that cFLN and cFLN-HpaA can stimulate macrophage cells to release Il-6 in a dose-depended manner as nature flagellin FliC isolated from S. typhimurium (Figure 3). The results suggested that refolded chimeric flagellin cFLN and fusion protein cFLN-HpaA restored protein conformation, restored the ability for activation of TLR5 and could effectively activate macrophage cells. However, activation ability of cFLN and cFLN-HpaA on macrophage cells might be weaker than that of FliC (Figure 3). Within cFLN, and cFLN-HpaA, D2, D3 domains of S. typhimurium FliC was replaced by D2, D3 domains in the flagellin of HP, D1 domain within cFLN, and cFLN-HpaA should maintain proper conformation and could recognized TLR 5 and activated macrophage[22]. However, D1 domain in the cFLN and cFLN-HpaA might be disturbed by D2 and D3 domain, affecting the interaction between TLR 5 and cFLN, or cFLN-HpaA. As a result, biological activity of cFLN, and cFLN-HpaA decreased compared with FliCs.

|
| Figure 3 Release of interleukin 6 from macrophages stimulated by flagellin, cFLN and cFLN-HpaA. Values are expressed as mean±S.D. (n=5). *: P < 0.05; **: P < 0.01; ***: P < 0.001. |
2.3 Characterization of antigen-loaded nanogels
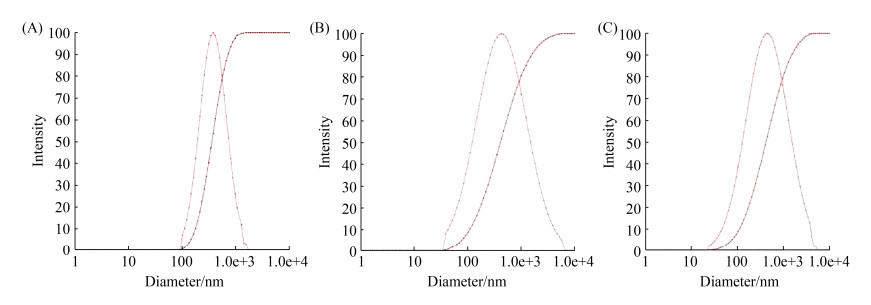
To enhance mucosal immune response to antigen, cFLN, HpaA and cFLN-HpaA were encapsulated within CS/TPP nanogels with an ionic gelation method[17]. Using the same process parameters, three kinds of nanogels, cFLN NGs, HpaA NGs, cFLN-HpaA NGs possessed similar encapsulation efficiency and drug loading (Table 2 and Figure 4). The particle size of nanogels closed to 500 nm (Table 2 and Figure 5), which were suitable for uptake by dentritic cells[23].
| Microparticles | Particle size/nm | DL/% | EE/% |
| HpaA NG | 380.6±20.6 | 0.10±0.03 | 57.6±6.6 |
| cFLN NG | 490.8±65.3 | 0.13±0.01 | 60.5±5.3 |
| cFLN-HpaA NG | 603.0±72.8 | 0.14±0.02 | 62.5±4.6 |
| Values are shown as mean±SD, n=3. EE and DL was determined by the method in section 2.6.1 in Materials and Methods. DL: drug loading; EE: encapsulation efficiency. | |||
|
| Figure 4 Size diameter of HpaA -loaded nanogels (A), cFLN-loaded nanogels (B), cFLN-HpaA-loaded nanogels (C) determined by a ZetaSizer nano ZS apparatus. |
|
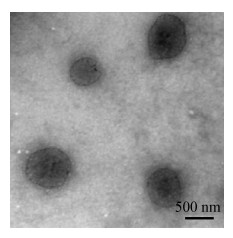
| Figure 5 TEM images of CS/TPP nanogels. |
2.4 In vivo studies
Effects of cFLN and nanogel encapsulation on hurmoral immune response induced by nasally delivered HpaA in mouse: The content of IgG, IgG1, IgG2a and IgA in the serum was investigated after nasal immunization and the results were shown in Figure 6. Both chimeric flagellin cFLN and nanogel encapsulation could effectively enhance humoral immune response induced by nasally delivered HpaA in mouse. The content of IgG, IgG1 and IgA induced by cFLN-HpaA were significantly higher than that induced by HpaA (P < 0.05, Figure 5). cFLN-HpaA induced 2.09, 1.4, and 2.62-fold increase in the content of IgG, IgG1 and IgA respectively compared to HpaA. The results indicated that cFLN might effectively enhance humoral immune responses induced by nasally delivered HpaA.

|
| Figure 6 Serum IgG, IgG1, IgG2a, IgA levels of BALB/c mice immunized with cFLN, HpaA and cFLN-HpaA solution or cFLN NG, HpaA NG and cFLN-HpaA NG. Values are expressed as mean±S.D. (n=5). Results over bars were compared with PBS. *: P < 0.05; **: P < 0.01; ***: P < 0.001. NS over bars indicated no significant difference, P > 0.05. |
The results further conformed that cFLN restored the ability for activation of TLR5 and possessed an intramolecular adjuvant effect to promote immune response induced by HPaA. These results were in line with the previous reports[16, 24]. Encapsulation of HpaA with nanogel particles further enhanced immune response induced by HpaA in mouse. cFLN NG, HpaA NG, and cFLN-HpaA NG produced 1.28 to 1.71-fold increase in the content of IgA, IgG, IgG1 and IgG2a in the serum compared to cFLN, HpaA, and cFLN-HpaA solution respectively (Figure 6). Moreover, most of these increases were statistically significant (Figure 6, P < 0.05). These results indicated CS/TPP NG could significantly enhance humoral immune response induced by nasally delivered HpaA, which was in line with previous reports[25-26]. CS NG has mucoadhesiveness and positive charge. These nature of CS NG contributes to improve transmucosal delivery of encapsulated antigen, uptake and processing of the encapsulated antigen by APC as well as a more efficient delivery to peripheral lymph nodes[23, 25].
Through calculation, it is found that the ratio of IgG1/IgG2a in the HpaA group is 2.6, the ratio of IgG1/IgG2a in the cFLN-HpaA group is 5.4, and the ratio of IgG1/IgG2a in the cFLN-HpaA NG group is 5.8. It can be seen that compared with the HpaA group, the ratio of IgG1/IgG2a in the cFLN-HpaA group was significantly increased. Compared with the cFLN-HpaA group, the ratio of IgG1/IgG2a in the cFLN-HpaA NG group also increased. It shows that cFLN and CS/TPP NG enhance Th2-type humoral immunity.
2.5 Effects of cFLN and nanogel encapsulation on local immune response of gastric mucosa induced by HpaA in mouseNasal vaccination could effectively induce immune responses in the nasopharynx-associated lymphoid tissue and mucosal immune responses in the gastric and the respiratory mucosa[11]. Thus, production of cytokines IFN-γ, IL-4 and IL-17, and mucosal antibodies sIgA in gastric mucosa were investigated after nasal administration of HpaA and the results were shown in Figure 6. Both chimeric flagellin cFLN and nanogel encapsulation could effectively enhance gastric mucosal immune response induced by HpaA in mouse. cFLN-HpaA solution induced 1.38, 1.16 and 1.58-fold increase in the content of IFN-γ, IL-4 and SIgA in gastric mucosa of mouse respectively compared with HpaA solution (P < 0.05, Figure 7). cFLN-HpaA NG produced 1.81, 1.71, 2.16 and 2.1-fold increase in the content of IFN-γ, IL-4, IL-17 and SIgA in gastric mucosa of mouse respectively compared with cFLN-HpaA solution (P < 0.05, Figure 7). SIgA can bind bacterial flagellum, might immobilize H. pylori and reduce H. pylori infection in the gut[27]. High levels of IFN-γ and IL-17 provide evidences for the strong Th1 and Th17 type immune response elicited by cFLN-HpaA and cFLN-HpaA NG. It has been demonstrated that Th1 and Th17 type immune response played a critical role in protecting against H. pylori infection in the stomach mucosa[28]. Therefore, cFLN-HpaA NG might be a promising nasal delivered vaccine system for protection against Helicobacter pylori infection.

|
| Figure 7 Production of IFN-γ, IL-4 and IL-17, and mucosal antibodies sIgA levels in gastric mucosal of BALB/c mice immunized with cFLN, HpaA and cFLN-HpaA solution or cFLN NG, HpaA NG and cFLN-HpaA NG. Values are expressed as mean±S.D. (n=5). Results over bars were compared with PBS. *: P < 0.05: **: P < 0.01; ***: P < 0.001. NS over bars indicated no significant difference, P > 0.05. |
3 Conclusion
In this study, chimeric flagellin cFLN was successfully constructed with D0, D1 domains of S. typhimurium flagellin FliC and D2, D3 domains of H. pylori flagellin FlaA. Used as intramolecular adjuvant, chimeric flagellin cFLN was linked with HpaA to construct a complex antigen cFLN-HpaA. cFLN, HpaA, cFLN-HpaA was expressed as inclusion body in recombinant E. coli and successfully renatured with the help of arginine and further purified by Ni-NTA Prepacked chromatographic column. Refolded chimeric flagellin cFLN and fusion protein cFLN-HpaA restored biologic activity, and could effectively activate macrophage cells. To enhance their immunogenicity, cFLN, HpaA, cFLN-HpaA were encapsulated into CS/TPP nanogels to prepare cFLN-HpaA NG, HpaA NG, cFLN-HpaA NG by an ionic gelation method. In vivo immunogenicity studies indicated that cFLN and CS/TPP nanogel encapsulation could effectively not only enhance hurmoral and mucosal immune responses induced by nasally delivered HpaA, but also effectively promote gastric mucosal immune response, Th1 and Th17 type immune response induced by nasally delivered HpaA in mouse. Therefore, these results suggested that cFLN-HpaA NG might be a promising nasally delivered vaccine system for protection against Helicobacter pylori infection.
| [1] | Salama NR, Hartung ML, Müller A. Life in the human stomach: persistence strategies of the bacterial pathogen Helicobacter pylori. Nature Reviews Microbiology, 2013, 11(6): 385-399. DOI:10.1038/nrmicro3016 |
| [2] | Flores-Treviño S, Mendoza-Olazarán S, Bocanegra-Ibarias P, Maldonado-Garza HJ, Garza-González E. Helicobacter pylori drug resistance: therapy changes and challenges. Expert Review of Gastroenterology & Hepatology, 2018, 12(8): 819-827. |
| [3] | Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, Rugge M, Selgrad M, Suerbaum S, Sugano K, El-Omar EM, European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the maastricht V/Florence consensus report. Gut, 2017, 66(1): 6-30. |
| [4] | Walduck A, Andersen LP, Raghavan S. Inflammation, immunity, and vaccines for Helicobacter pylori infection. Helicobacter, 2015, 20: 17-25. DOI:10.1111/hel.12252 |
| [5] | Carlsohn E, Nyström J, Bölin I, Nilsson CL, Svennerholm AM. HpaA is essential for Helicobacter pylori colonization in mice. Infection and Immunity, 2006, 74(2): 920-926. DOI:10.1128/IAI.74.2.920-926.2006 |
| [6] | Ghosh P, Bhakta S, Bhattacharya M, Sharma AR, Sharma G, Lee SS, Chakraborty C. A novel multi-epitopic peptide vaccine candidate against Helicobacter pylori: in-silico identification, design, cloning and validation through molecular dynamics. International Journal of Peptide Research and Therapeutics, 2021, 27(2): 1149-1166. DOI:10.1007/s10989-020-10157-w |
| [7] | Nyström J, Svennerholm AM. Oral immunization with HpaA affords therapeutic protective immunity against H. pylori that is reflected by specific mucosal immune responses. Vaccine, 2007, 25(14): 2591-2598. DOI:10.1016/j.vaccine.2006.12.026 |
| [8] | Flach CF, Svensson N, Blomquist M, Ekman A, Raghavan S, Holmgren J. A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine, 2011, 29(6): 1235-1241. DOI:10.1016/j.vaccine.2010.11.088 |
| [9] | Boyaka PN. Inducing mucosal IgA: a challenge for vaccine adjuvants and delivery systems. Journal of Immunology, 2017, 199(1): 9-16. DOI:10.4049/jimmunol.1601775 |
| [10] | Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nature Reviews Immunology, 2012, 12(8): 592-605. DOI:10.1038/nri3251 |
| [11] | Dai XJ, He JT, Zhang RX, Wu GH, Xiong FF, Zhao BH. Co-delivery of polyinosinic: polycytidylic acid and flagellin by poly(lactic-co-glycolic acid) MPs synergistically enhances immune response elicited by intranasally delivered hepatitis B surface antigen. International Journal of Nanomedicine, 2017, 12: 6617-6632. DOI:10.2147/IJN.S146912 |
| [12] | Moyle PM. Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines. Biotechnology Advances, 2017, 35(3): 375-389. DOI:10.1016/j.biotechadv.2017.03.005 |
| [13] | Mizel SB, Bates JT. Flagellin as an adjuvant: cellular mechanisms and potential. Journal of Immunology, 2010, 185(10): 5677-5682. DOI:10.4049/jimmunol.1002156 |
| [14] | Hajam IA, Dar PA, Shahnawaz I, Jaume JC, Lee JH. Bacterial flagellin-a potent immunomodulatory agent. Experimental & Molecular Medicine, 2017, 49(9): e373. |
| [15] | Mori J, Vranac T, Smrekar B, Černilec M, Šerbec VČ, Horvat S, Ihan A, Benčina M, Jerala R. Chimeric flagellin as the self-adjuvanting antigen for the activation of immune response against Helicobacter pylori. Vaccine, 2012, 30(40): 5856-5863. |
| [16] | González MJ, Iribarnegaray V, Zunino P, Scavone P. Purification of native flagellin. Methods in Molecular Biology: Clifton, N J, 2019, 2021: 35-44. |
| [17] | Jonassen H, Kjøniksen AL, Hiorth M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules, 2012, 13(11): 3747-3756. DOI:10.1021/bm301207a |
| [18] | Flach CF, Svensson N, Blomquist M, Ekman A, Raghavan S, Holmgren J. A truncated form of HpaA is a promising antigen for use in a vaccine against Helicobacter pylori. Vaccine, 2011, 29(6): 1235-1241. |
| [19] | He JT, Wang GZ, Xu RG, Feng JL, Wang JL, Su HB, Song HY. Refolding of a staphylokinase variant Y1-sak by reverse dilution. Applied Biochemistry and Biotechnology, 2008, 151(1): 29-41. |
| [20] | Yamaguchi H, Miyazaki M. Refolding techniques for recovering biologically active recombinant proteins from inclusion bodies. Biomolecules, 2014, 4(1): 235-251. |
| [21] | Tsumoto K, Umetsu M, Kumagai I, Ejima D, Philo JS, Arakawa T. Role of arginine in protein refolding, solubilization, and purification. Biotechnology Progress, 2004, 20(5): 1301-1308. |
| [22] | Smith KD, Andersen-Nissen E, Hayashi F, Strobe K, Bergman MA, Rassoulian Barrett SL, Cookson BT, Aderem A. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nature Immunology, 2003, 4(12): 1247-1253. |
| [23] | Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. International Journal of Pharmaceutics, 2005, 298(2): 315-322. |
| [24] | Bennett KM, Gorham RD, Gusti V, Trinh L, Morikis D, Lo DD. Hybrid flagellin as a T cell independent vaccine scaffold. BMC Biotechnology, 2015, 15: 71. |
| [25] | Amidi M, Romeijn SG, Verhoef JC, Junginger HE, Bungener L, Huckriede A, Crommelin DJA, Jiskoot W. N-trimethyl chitosan (TMC) nanoparticles loaded with influenza subunit antigen for intranasal vaccination: biological properties and immunogenicity in a mouse model. Vaccine, 2007, 25(1): 144-153. |
| [26] | Prego C, Paolicelli P, Díaz B, Vicente S, Sánchez A, González-Fernández Á, Alonso MJ. Chitosan-based nanoparticles for improving immunization against hepatitis B infection. Vaccine, 2010, 28(14): 2607-2614. |
| [27] | Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nature Reviews Immunology, 2012, 12(8): 592-605. |
| [28] | Cullender TC, Chassaing B, Janzon A, Kumar K, Muller CE, Werner JJ, Angenent LT, Bell ME, Hay AG, Peterson DA, Walter J, Vijay-Kumar M, Gewirtz AT, Ley RE. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host & Microbe, 2013, 14(5): 571-581. |
 2021, Vol. 61
2021, Vol. 61




