
中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 毛明雨, 张若凡, 黄国文, 李轩, 成艳芬, 余凯凡, 朱伟云. 2021
- Mingyu Mao, Ruofan Zhang, Guowen Huang, Xuan Li, Yanfen Cheng, Kaifan Yu, Weiyun Zhu. 2021
- 猪源乳杆菌Lactobacillus reuteri strain DSM和Lactobacillus taiwanensis strain BCRC对肠道炎症的影响
- Effects of Lactobacillus reuteri strain DSM and Lactobacillus taiwanensis strain BCRC on intestinal inflammation
- 微生物学报, 61(11): 3715-3725
- Acta Microbiologica Sinica, 61(11): 3715-3725
-
文章历史
- 收稿日期:2021-02-20
- 修回日期:2021-03-19
- 网络出版日期:2021-06-05
乳酸杆菌作为一类常见益生菌,在食品工业和畜牧业具有广泛的应用前景。单胃动物消化道中乳酸杆菌属是优势均属,其在调节机体肠道内环境稳态方面具有重要作用,如参与宿主肠道屏障、免疫应答以及营养代谢等[1–3]。研究表明,一些乳酸杆菌能改善肠道炎症。Preston等研究发现,鼠李糖乳杆菌CLR2、干酪乳杆菌LBC80R和嗜酸乳杆菌CL1285能够有效缓解肠应激综合征患者相关症状[4]。乳酸杆菌对肠道炎症的作用机制并不明确,目前其可能的途径主要有:降低肠道中的pH值、调节肠道内环境、改善肠黏膜屏障、维持肠道菌群平衡、减少病原菌定殖等。另有研究表明,乳杆菌产生的特定代谢物在调节肠道炎症过程中也发挥重要作用。例如,Ma等研究发现戊糖乳杆菌显著增加吲哚丙酮酸及泛酸水平,抑制DSS诱导的小鼠结肠炎,改善结肠损伤[5]。Hou等研究表明,罗伊氏乳杆菌D8可以促进肠道干细胞再生加速肠黏膜损伤的修复,在此过程中吲哚- 3-醛刺激LPL生成IL-22,诱导肠上皮细胞STAT3磷酸化[6]。近年来,色氨酸菌群代谢物的功能受到广泛关注,吲哚及其衍生物被发现可调节肠上皮屏障,改善DSS诱导的肠道炎症[7–9]。因此,本实验室前期从健康猪粪样中分离乳杆菌属细菌,并基于代谢色氨酸的能力筛选了菌株,得到2株乳杆菌Lactobacillus reuteri strain DSM与Lactobacillus taiwanensis strain BCRC。本研究旨在以DSS诱导小鼠肠炎模型研究该猪源乳杆菌对肠道炎症的影响,为后期潜在应用提供指导。
1 材料和方法 1.1 菌株及其培养条件本实验室分离自健康猪粪样的2株乳杆菌Lactobacillus reuteri strain DSM(LR)与Lactobacillus taiwanensis strain BCRC (LT)。从–20 ℃冰箱中取出所保存的乳杆菌菌株,于37 ℃复苏,之后按照2%的接种量接种至10 mL的液体MRS培养基中,培养16 h取出,涂板至固体MRS培养基,挑取单菌落进行纯化培养。然后,采用纯化后的菌液扩大培养,取50 µL菌液至10 mL培养基,培养好后取出,在5000 r/min、4 ℃条件离心15 min后弃掉上清液,用PBS洗涤3次,再加入适量的生理盐水混匀,调整乳杆菌的浓度,根据小鼠体重计算出给菌量。
1.2 动物试验试验用C57BL/6J小鼠购自江苏省南京市青龙山动物繁育场。选取体重相近的雄鼠24只,随机分为4组:正常对照组(Normal)、DSS组(DSS)、LR菌处理组(DSS+LR)和LT菌处理组(DSS+LT),每组6只小鼠,单笼饲养。适应期7 d,试验期16 d。试验第0–13天,正常对照组和DSS组每天灌胃200 μL生理盐水,LR、LT菌处理组每天分别灌胃200 μL含5×108 CFU乳杆菌LR和LT的生理盐水。第7–13天,DSS组、LR菌处理组和LT菌处理组在饮水中添加3% DSS,正常对照组正常饮水。第13–16天,4组小鼠均正常饮水,随后处死小鼠并采样。试验期饮水每2天更换1次,每天记录小鼠体重,观察小鼠活动和粪便情况。采集血液样本、结肠组织、结肠食糜用于后续分析,测量结肠长度。
1.3 疾病活动指数(disease activity index,DAI) 评分DAI评分参考Cooper等的评分标准[10],包括3个方面:体重变化、大便形状和便血情况。体重下降0%,大便形状正常,血便正常,0分;体重下降1–5%、5–10%、10–15%,大便形状松散,隐血阳性分别计1、2、3分;体重下降 > 15%,稀便,肉眼血便,计4分。
1.4 结肠组织切片及H & E染色取结肠组织(约1 cm),4%多聚甲醛固定1 d,用水冲洗后进行脱水、透明、浸蜡从而将组织样品包埋于石蜡中。然后进行切片和制片,用苏木精和伊红(H & E)染色,封片。使用共聚焦光学显微镜观察、拍照。
1.5 炎症因子和髓过氧化物酶(myeloperoxidase,MPO)的测定结肠组织和血浆炎症因子TNF-ɑ、IL-1β、IL-6和IL-10浓度使用猪ELISA试剂盒(南京建成生物技术有限公司)测定,实验步骤参照试剂盒说明书。结肠组织MPO活性使用ELISA试剂盒(北京方程生物科技有限公司)参照试剂盒说明书进行测定。
1.6 肠上皮屏障相关蛋白表达的测定免疫组化法检测结肠组织Claudin-1、Occludin、ZO-1和Muc-2表达在武汉赛维尔生物科技有限公司进行,实验简要步骤:切片脱蜡后进行抗原修复,3%双氧水处理,画圈,血清封闭后一抗4 ℃孵育过夜,二抗室温孵育后显色,苏木精染核,脱水、透明、封片,显微镜检查拍照。ELISA法测定Claudin-1、Occludin、ZO-1和Muc-2表达,使用相应试剂盒(北京方程生物科技有限公司)参照试剂盒说明书进行测定。
1.7 结肠食糜总菌DNA的提取及16S rDNA微生物测序参照Zoetendal等[11]进行食糜总基因组DNA提取,用磁珠法破碎,苯酚-氯仿萃取。选择通用的特异性引物对16S rDNA的V3–V4区进行扩增,产物纯化进行等量混合,构建测序文库,在Hiseq2500(PE250)平台测序。原始数据经筛选和质控,获得有效功能分类单元(OTU)。通过Omicsmart在线分析系统(http://www.omicsmart.com)分析数据,进行OTU注释、菌群门水平和属水平相对丰度分析、加权和未加权UniFrac距离的主坐标分析(PCoA)。
1.8 数据统计分析试验数据采用SPSS 20.0软件(SPSS Inc.,Chicago,IL,USA)进行统计分析,ANOVA单因素方差分析各组间差异,结果用平均值±标准误(Mean±SEM)表示;当P < 0.05时,表示差异显著。数据图片使用Graphpad Prism 8.0软件(La Jolla,CA,USA)制作。
2 结果和分析 2.1 乳杆菌对DSS诱导结肠炎小鼠体重、DAI评分、结肠长度的影响建立DSS诱导小鼠结肠炎模型,评估猪源乳杆菌Lactobacillus reuteri strain DSM(LR)与Lactobacillus taiwanensis strain BCRC(LT)对小鼠肠炎的影响。结果显示,与正常对照组相比,DSS组小鼠体重、结肠长度显著降低,DAI评分显著增加(P < 0.05,图 1),表明DSS诱导成功构建了结肠炎模型。与DSS组相比,LR和LT菌处理组小鼠体重下降的程度并没有显著缓解,但是,其DAI评分显著降低;并且,LT菌处理组结肠长度显著增加(P < 0.05)。

|
| 图 1 乳杆菌对DSS诱导结肠炎小鼠体重、DAI评分、结肠长度的影响 Figure 1 Effects of Lactobacillus on the body weight, DAI scores and colon length in DSS-induced colitis. A: schematic depicting the design of the animal experiment; B: the body weights of mice in the four groups recorded from day 0 to day 16 are plotted and presented as mean (SE); C: the disease activity index (DAI) scores after DSS administration from day 8 to day 12; D: representative images of the colon in mice; E: the colon length on day 16. *P < 0.05 according to one-way ANOVA analysis. |
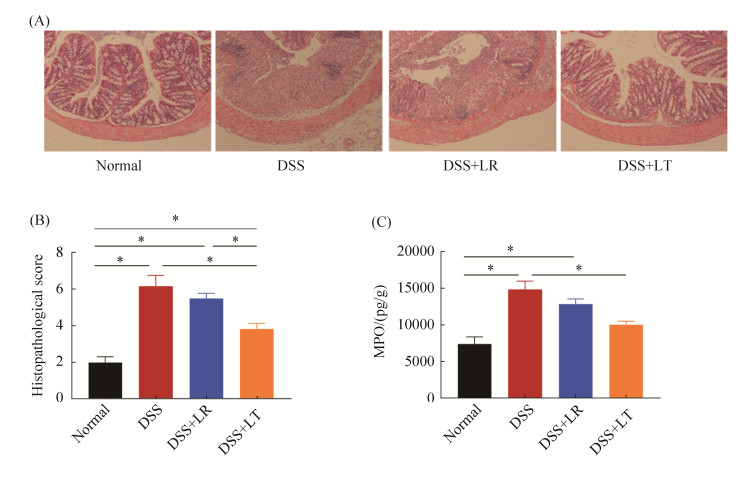
2.2 乳杆菌对DSS诱导结肠炎小鼠肠道组织形态、病理学评分、MPO活性的影响
小鼠结肠组织切片HE染色、组织学评分、MPO结果见图 2。正常对照组和LT菌处理组结肠组织排列整齐,隐窝正常,杯状细胞无减少,未见黏膜糜烂、出血;而DSS组和LR菌处理组结肠组织隐窝破坏变形,黏膜糜烂、出血,上皮细胞破坏(图 2-A)。对每只小鼠的结肠切片进行组织病理学评分并进行统计,结果显示,正常对照组和LT菌处理组结肠组织病理学评分显著低于DSS组和LR菌处理组(P < 0.05,图 2-B)。髓过氧化物酶(MPO)是血红素过氧化物酶超家族的成员,其活性变化代表嗜中性多形核白细胞的功能状态,反映炎症严重情况。结果显示,DSS组结肠MPO水平显著高于正常对照组,LT菌处理能显著降低MPO水平(P < 0.05,图 2-C)。
|
| 图 2 乳杆菌对DSS诱导结肠炎小鼠肠道组织形态、病理学评分、MPO活性的影响 Figure 2 Effects of Lactobacillus on the intestinal morphology (A), histopathological scores (B) and MPO activity (C) in DSS-induced colitis. *P < 0.05 according to one-way ANOVA analysis. |
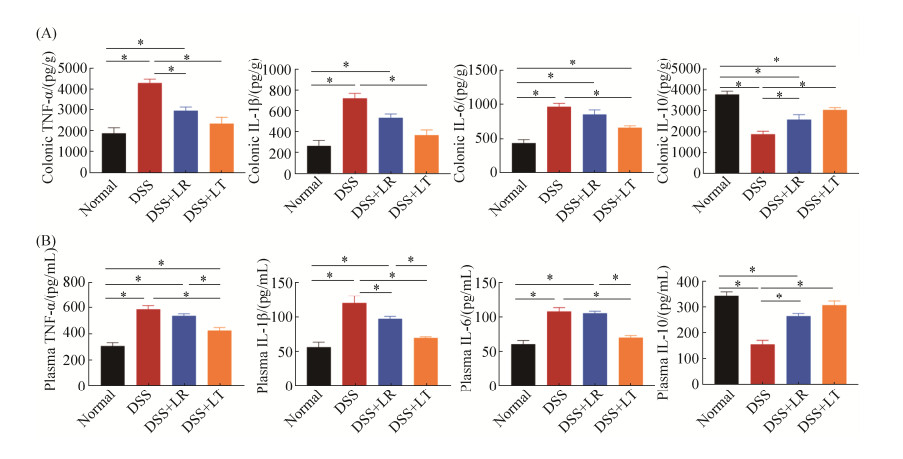
2.3 乳杆菌对DSS诱导结肠炎小鼠炎症因子的影响
小鼠结肠组织和血浆的炎症因子浓度见图 3。与正常对照组相比,DSS组结肠组织促炎因子TNF-α、IL-1β、IL-6的水平显著升高,而抗炎因子IL-10水平显著降低(P < 0.05)。与DSS组相比,LT菌处理组结肠组织TNF-α、IL-1β、IL-6的水平显著降低,IL-10水平显著升高;LR菌处理组结肠组织TNF-α水平显著降低,IL-10水平显著升高(P < 0.05),IL-1β和IL-6水平变化无显著差异(P > 0.05)。与结肠的炎症因子结果相似,正常对照组和LT菌处理组血浆的促炎因子TNF-α、IL-1β、IL-6的水平显著低于DSS组,而抗炎因子IL-10水平显著高于DSS组;LR菌处理组血浆IL-1β水平显著低于DSS组,IL-10水平显著高于DSS组,TNF-α和IL-6水平无显著变化(P > 0.05)。
|
| 图 3 乳杆菌对DSS诱导结肠炎小鼠肠道和血浆炎症因子的影响 Figure 3 Effects of Lactobacillus on the concentrations of the intestinal and plasma inflammatory factors. A: the colonic levels of the cytokines TNF-α, IL-1β, IL-6 and IL-10 in the four groups; B: the plasma levels of the cytokines. *P < 0.05 according to one-way ANOVA analysis. |
2.4 乳杆菌对DSS诱导结肠炎小鼠肠上皮屏障相关蛋白表达的影响
免疫组化和ELISA方法检测小鼠结肠组织紧密连接蛋白、黏蛋白表达的结果如图 4所示。相比于正常对照组,DSS降低了结肠紧密连接蛋白Claudin-1、Occludin、ZO-1和黏蛋白Muc-2表达水平,而LR和LT菌的处理则上调了DSS影响下的屏障相关蛋白表达(图 4-A、B)。ELISA方法对这些蛋白的定量结果印证了免疫组化结果,其中LR、LT菌处理显著上调Claudin-1、ZO-1的表达水平,LT菌处理还显著上调了Occludin的表达水平(P < 0.05)。

|
| 图 4 乳杆菌对DSS诱导结肠炎小鼠肠上皮屏障相关蛋白表达的影响 Figure 4 Effects of Lactobacillus on the expression of the intestinal epithelial barrier related proteins. A: representative images of Claudin-1, Occludin, ZO-1 and Muc-2 immunohistochemical staining; B: mean of IOD according to the immunohistochemical staining; C: the colonic levels of Claudin-1, Occludin, ZO-1 and Muc-2 measured by ELISA. *P < 0.05 according to one-way ANOVA analysis. |
2.5 乳杆菌对DSS诱导结肠炎小鼠肠道菌群的影响
利用16S rDNA测序技术检测了结肠内容物的菌群组成。PCoA和PCA分析结果均显示,正常对照组和DSS组显著分离,形成两个不同的集群(P < 0.001,图 5-A、5-B);LR、LT菌处理组与DSS组呈现一定程度的分离。并且,LT处理组和正常对照组在坐标上偏移趋向更接近,提示LT处理组菌群组成更接近于正常对照组。

|
| 图 5 乳杆菌对DSS诱导结肠炎小鼠肠道菌群影响的PCoA分析 Figure 5 PCoA analysis of the effects of Lactobacillus on the intestinal flora in DSS-induced colitis. PCoA and PCA plot based on the weighted and unweighted UniFrac distances among the four groups. Each point represents a sample. |
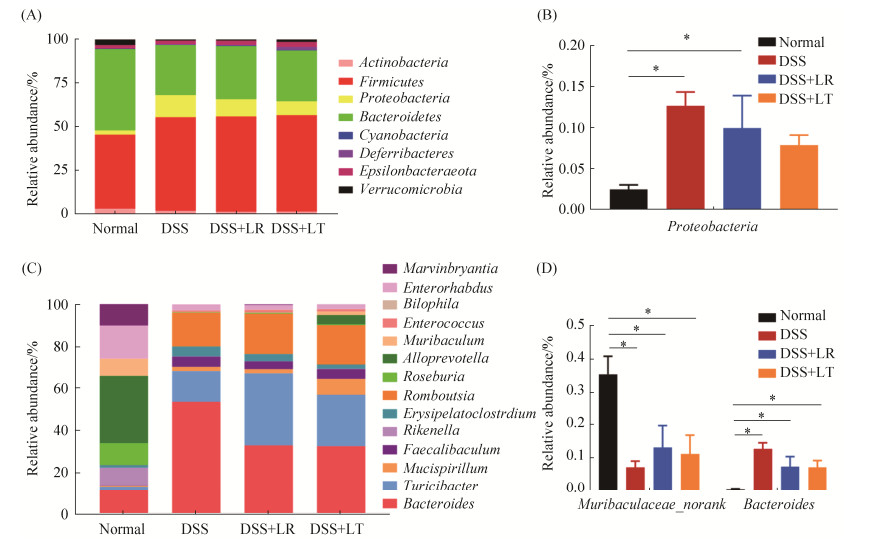
在门水平上,4组小鼠结肠菌群均以厚壁菌门、拟杆菌门、变形菌门为优势菌门(图 6-A)。其中,变形菌门丰度在正常对照组显著低于DSS组(P < 0.05);与DSS组相比,LT菌处理组也能降低变形菌门丰度,但未达显著水平(P > 0.05)(图 6-B)。在属水平上,正常对照组优势属丰度与其他3组呈现明显不同的模式(图 6-C)。其中,Muribaculaceae_norank属在正常对照组显著高于其他3组,Bacteroides在正常对照组显著低于其他3组(P < 0.05);与DSS组相比,LR和LT菌的处理增加了Muribaculaceae_norank丰度,降低了Bacteroides菌丰度,但差异不显著(图 6-D)。
|
| 图 6 乳杆菌对DSS诱导结肠炎小鼠肠道菌群门水平和属水平丰度的影响 Figure 6 Effects of Lactobacillus on the phylum level and genus level abundance in DSS-induced colitis. Relative abundance of reads at phylum-level (A) and significantly different phyla (B), and relative abundance of reads at genus-level (C) and significantly different genera (D). *P < 0.05. |
3 讨论
本研究以DSS诱导小鼠肠炎模型研究了2株乳杆菌Lactobacillus reuteri strain DSM与Lactobacillus taiwanensis strain BCRC对肠道炎症的影响。研究发现,该两株乳杆菌对DSS引起的小鼠体重下降没有显著缓解作用,但DAI评分和病理学评分得到改善,LT菌增加了结肠长度、降低了MPO活性。这些结果表明了两株乳杆菌尤其是乳杆菌Lactobacillus reuteri一定程度上缓解了肠道炎症。
乳酸杆菌缓解肠道炎症的机理可能包括增强肠道紧密连接蛋白表达、提升抗氧化状态、降低促炎细胞因子、增加抗炎细胞因子释放、调节调节性T细胞和巨噬细胞等[12–14],但确切的分子机制仍然未知。本研究中,LT乳杆菌降低了结肠和血浆的促炎因子TNF-α、IL-1β、IL-6水平,升高了抗炎IL-10水平。LR菌则可降低结肠TNF-α水平、血浆IL-1β水平,升高IL-10水平。某些乳杆菌的抗炎特性也被其他研究报道。例如,Pan等研究表明,Lactobacillus murinus在能量限制小鼠中丰度提高,减轻炎症[15]。Li等研究发现,Lactobacillus rhamnosus strain在体内和体外均能抵抗LPS诱导的肠道炎症[16]。
乳杆菌对炎症因子分泌的影响很可能是通过其增强肠上皮屏障功能来实现。Jang等就报道了发酵乳杆菌株增强紧密连接蛋白表达,减缓DSS诱导的肠道炎症[17]。本研究中,DSS的处理降低了结肠紧密连接蛋白Claudin-1、Occludin、ZO-1和黏蛋白Muc-2的表达,而LT菌则能上调这些屏障相关蛋白的表达,LR菌部分影响这些蛋白的表达。这些结果印证了LT菌有良好的缓解肠道炎症的效果。
益生菌对宿主的保护作用还可能是通过其影响肠道菌群组成及其代谢来进行。Jang等研究发现,发酵乳杆菌KBL374、KBL375增加了肠道菌群的多样性,尤其是KBL375能增加有益菌如乳杆菌属和Akkermansia spp.的数量[17]。在本研究中,PCoA分析显示了正常对照组、DSS组以及两株乳杆菌的分别处理组显著区分,并且LT菌处理组与正常对照组更接近。门水平上,DSS显著增加了变形菌门的丰度;属水平上,Muribaculaceae_ norank属降低,Bacteroides属升高。LT菌的处理降低了变形菌门丰度,增加了Muribaculaceae_ norank丰度,降低了Bacteroides菌丰度,但在统计学上未达显著水平,可能与测序样本数量有限有关。拟杆菌属(Bacteroides)已被报道与IBD疾病活动程度有关[18]。乳杆菌的代谢产物如乳酸、短链脂肪酸,被认为在维持机体健康中发挥重要作用,例如维持肠道内环境、充当肠道上皮营养组分、保护肠黏膜屏障等。色氨酸菌群代谢物如吲哚-3-醛、吲哚-3-丙酸可调节肠上皮屏障,改善肠道炎症[8–9]。本研究使用的2株乳杆菌是前期基于有较强代谢色氨酸的能力情况下进行筛选的。在本试验中,乳杆菌是否由其代谢产物发挥改善肠道炎症的作用还有待进一步研究。
综上所述,乳杆菌Lactobacillus reuteri与Lactobacillus taiwanensis可增强肠上皮屏障相关蛋白表达,降低促炎因子水平、增加抗炎因子水平等,一定程度缓解肠道炎症;其中,Lactobacillus taiwanensis对肠上皮屏障的保护效果更为明显。
| [1] | O'Hara AM, O'Regan P, Fanning Á, O'Mahony C, MacSharry J, Lyons A, Bienenstock J, O'Mahony L, Shanahan F. Functional modulation of human intestinal epithelial cell responses by Bifidobacterium infantis and Lactobacillus salivarius. Immunology, 2006, 118(2): 202-215. DOI:10.1111/j.1365-2567.2006.02358.x |
| [2] | Qin HL, Shen TY, Gao ZG, Fan XB, Hang XM, Jiang YQ, Zhang HZ. Effect of Lactobacillus on the gut microflora and barrier function of the rats with abdominal infection. World Journal of Gastroenterology, 2005, 11(17): 2591-2596. DOI:10.3748/wjg.v11.i17.2591 |
| [3] | Wang W, Chen LP, Zhou R, Wang XB, Song L, Huang S, Wang G, Xia B. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. Journal of Clinical Microbiology, 2014, 52(2): 398-406. DOI:10.1128/JCM.01500-13 |
| [4] | Preston K, Krumian R, Hattner J, de Montigny D, Stewart M, Gaddam S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: a double-blind, randomised, placebo-controlled study. Beneficial Microbes, 2018, 9(5): 697-706. DOI:10.3920/BM2017.0105 |
| [5] | Ma Y, Hu C, Yan WX, Jiang HM, Liu G. Lactobacillus pentosus increases the abundance of Akkermansia and affects the serum metabolome to alleviate DSS-induced colitis in a murine model. Frontiers in Cell and Developmental Biology, 2020, 8: 591408. DOI:10.3389/fcell.2020.591408 |
| [6] | Hou QH, Ye LL, Liu HF, Huang LL, Yang Q, Turner J, Yu QH. Lactobacillus accelerates ISCs regeneration to protect the integrity of intestinal mucosa through activation of STAT3 signaling pathway induced by LPLs secretion of IL-22. Cell Death & Differentiation, 2018, 25(9): 1657-1670. |
| [7] | Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nature Communications, 2018, 9: 3294. DOI:10.1038/s41467-018-05470-4 |
| [8] | Scott SA, Fu JJ, Chang PV. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(32): 19376-19387. DOI:10.1073/pnas.2000047117 |
| [9] | Li JJ, Zhang L, Wu T, Li YF, Zhou XJ, Ruan Z. Indole-3-propionic acid improved the intestinal barrier by enhancing epithelial barrier and mucus barrier. Journal of Agricultural and Food Chemistry, 2021, 69(5): 1487-1495. DOI:10.1021/acs.jafc.0c05205 |
| [10] | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Laboratory Investigation, 1993, 69(2): 238-249. |
| [11] | Zoetendal EG, Akkermans ADL, de Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Applied and Environmental Microbiology, 1998, 64(10): 3854-3859. DOI:10.1128/AEM.64.10.3854-3859.1998 |
| [12] | Gao CX, Major A, Rendon D, Lugo M, Jackson V, Shi ZC, Mori-Akiyama Y, Versalovic J. Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. mBio, 2015, 6(6): e01358-e01315. |
| [13] | Dogi C, García G, de Moreno de LeBlanc A, Greco C, Cavaglieri L. Lactobacillus rhamnosus RC007 intended for feed additive: immune-stimulatory properties and ameliorating effects on TNBS-induced colitis. Beneficial Microbes, 2016, 7(4): 539-547. DOI:10.3920/BM2015.0147 |
| [14] | Eun SH, Lim SM, Jang SE, Han MJ, Kim DH. Lactobacillus sakei K17, an inducer of IL-10 expression in antigen-presenting cells, attenuates TNBS-induced colitis in mice. Immunopharmacology and Immunotoxicology, 2016, 38(6): 447-454. DOI:10.1080/08923973.2016.1233981 |
| [15] | Pan FW, Zhang LY, Li M, Hu YX, Zeng BH, Yuan HJ, Zhao LP, Zhang CH. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome, 2018, 6(1): 54. DOI:10.1186/s40168-018-0440-5 |
| [16] | Li XS, Hu D, Tian YZ, Song Y, Hou YC, Sun LL, Zhang Y, Man CX, Zhang W, Jiang YJ. Protective effects of a novel Lactobacillus rhamnosus strain with probiotic characteristics against lipopolysaccharide-induced intestinal inflammation in vitro and in vivo. Food & Function, 2020, 11(7): 5799-5814. |
| [17] | Jang YJ, Kim WK, Han DH, Lee K, Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes, 2019, 10(6): 696-711. DOI:10.1080/19490976.2019.1589281 |
| [18] | Zhou YT, Zhi FC. Lower level of Bacteroides in the gut microbiota is associated with inflammatory bowel disease: a meta-analysis. BioMed Research International, 2016, 2016: 5828959. |
 2021, Vol. 61
2021, Vol. 61




