中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- 周连玉, 焦璐, 巨家升, 马学兰, 蒋霞, 乔枫. 2021
- Lianyu Zhou, Lu Jiao, Jiasheng Ju, Xuelan Ma, Xia Jiang, Feng Qiao. 2021
- 硒与内生真菌对中华羊茅生长和代谢产物的影响
- Effects of Se and Epichloë endophyte on the growth and metabolic compounds of Festuca sinensis
- 微生物学报, 61(11): 3726-3743
- Acta Microbiologica Sinica, 61(11): 3726-3743
-
文章历史
- 收稿日期:2021-02-20
- 修回日期:2021-04-25
- 网络出版日期:2021-09-09
硒在植物体内发挥着重要的生理生化作用,如促生长发育[1]、改变代谢产物[2]。最近有研究表明微生物影响植物生长、吸收硒、硒代谢及产生的代谢物[3-5]。乔亚君等[6]发现在一定施硒水平下接种丛枝菌根真菌促进丹参营养和药用成分的积累。
冷季牧草如多年生黑麦草、高羊茅、草地羊茅,常常感染内生真菌Epichloë[7-8],这些内生真菌的有些种类曾被作为Neotyphodium spp.[9]。许多禾草-内生真菌共生体的研究表明内生真菌Epichloë赋予宿主许多益处[10],包括促进生长、提高营养吸收能力[11]、增强对非生物或生物胁迫的抵抗力[12-15],而基于代谢组学对禾草-内生真菌共生体互作关系的研究报道并不多见。Rasmussen等[16]使用气相色谱-质谱联用技术(GC-MS)检测两种黑麦草在不同氮肥水平下的有机酸、糖醇类、脂肪酸等66种代谢产物,与不带内生真菌Epichloë (E–)植株相比,带内生真菌Epichloë (E+)植株中有44种代谢产物含量在不同氮肥水平下存在差异;施加高浓度氮肥促进有机酸和脂肪酸含量上升;内生真菌感染引起植株中某些含氮化合物含量的下降,而碳水化合物尤其是蔗糖、脂肪酸、有机酸(奎尼酸、莽草酸)和酚类含量增加。Nagabhyru等[17]采用离子交换色谱法和液相色谱-质谱联用技术(LC-MS)对干旱胁迫下的3个高羊茅株系E+和E–进行了研究,在干旱胁迫2–3 d,内生真菌能增加植物地上部分和根系中葡萄糖、果糖、海藻糖、糖醇、脯氨酸和谷氨酸含量,且内生真菌代谢物甘露醇和黑麦草碱水平也在干旱胁迫下呈上升趋势。
近年来对中华羊茅-内生真菌共生体已进行了多方面的研究,报道了中华羊茅种群带菌率、菌株的生物学与生理学特性、形态多样性、系统发育关系及生物活性[18-24]。中华羊茅内生真菌也属于香柱菌(Epichloё)[9, 21],能促进中华羊茅植株生长[25-26],并提高抵抗干旱、水涝、低温、盐分、重金属等胁迫的能力[27-32]。在上述研究的基础上,本试验就中华羊茅两个地理种群响应亚硒酸钠后其生长指标和代谢产物的变化进行研究,以期深入理解内生真菌和硒对宿主代谢的影响。
1 材料和方法 1.1 植物材料分别在青海海北(97°05′ E,36°47′ N,3700 m,HB)和青海玉树(97°08′ E,33°21′ N,3904 m,YS)地方采获中华羊茅植株种子,4 ℃保存于青海师范大学微生物实验室。
1.2 方法 1.2.1 建立中华羊茅地理种群E+、E–及处理:将中华羊茅共生体(E+)种子用甲基托布津处理以获得非共生体(E–)种群[33]。E+和E–种子于湿润的滤纸上进行萌发,然后将幼苗移栽装有灭菌基质(普通土壤: 营养土: 河砂,1:1:1,V/V/V)的塑料花盆(上径16 cm×底径9 cm×高11 cm)中。根据植物需水情况进行日常管理。利用苯胺蓝染色检测4周龄幼苗的带菌情况[34],建立E+和E–种群。在花盆中装入750.0 g灭菌河沙,以E+、E–植株的分蘖进行分株繁殖,每盆2株幼苗,一个月后以亚硒酸钠浓度为20 mg/L的改良营养液[Ca(NO3)2 0.945 g/L,KNO3 0.506 g/L,NH4NO3 0.08 g/L,KH2PO4 0.136 g/L,MgSO4 0.493 g/L,FeSO4 0.0139 g/L,EDTA-Na 0.01865 g/L]作为Se处理,对照组(CK)为改良营养液,每个处理10盆,每周浇灌100 mL,浇灌7周后测量生长指标后,迅速收集样品放入液氮罐中,贮存于–20 ℃,用于代谢产物分析。
1.2.2 生长指标测定:取样前测量株高和分蘖数,将收获的植株分成地上部分和根系,用清水冲洗根系,测定根长,并用游标卡尺测量根直径。
1.2.3 代谢组学样品提取与衍生化方法:称取样本(10±1) mg于1.5 mL离心管中,依次加入450 μL预冷甲醇(甲醇: 水=3:1,V/V)和10 μL核糖醇,涡旋30 s;加入钢珠,35 Hz研磨仪处理4 min,超声5 min (冰水浴),将样品于4 ℃下10000 r/min离心15 min。将200 μL上清液移至1.5 mL离心管中,每个样本各取60 μL混合成QC样本,在真空浓缩器中干燥提取物。向干燥后的代谢物加入60 μL甲氧胺盐试剂(甲氧胺盐酸盐,溶于吡啶20 mg/mL),轻轻混匀后,放入烘箱中80 ℃孵育30 min;之后向每个样品中加入80 μL BSTFA (含有1% TMCS,V/V),将混合物70 ℃孵育1.5 h;冷却至室温,向混合的样本中加入5 μL FAMEs (溶于氯仿),用于GC-MS上机分析。
1.2.4 GC-MS分析及代谢数据比对:Agilent 7890气相色谱-飞行时间质谱联用仪配有Agilent DB-5MS毛细管柱(30 m×250 μm×0.25 μm,J & W Scientific,Folsom,CA,USA)。色谱条件:柱初温50 ℃,保持1 min,以10 ℃/min升温速度升至310 ℃,保持8 min。载气为高纯氮气,流速1 mL/min,进样量1.0 μL,不分流。质谱条件:EI离子源,电离电压70 eV,离子源温度250 ℃,前进样口温度280 ℃,传输线温度280 ℃,扫描速率12.5/s,溶剂延迟6.25 min,质量范围50–500 amu。
使用ChromaTOF软件(V 4.3x,LECO)对质谱数据进行了峰提取、基线矫正、解卷积、峰积分、峰对齐等分析。使用了LECO-Fiehn Rtx5数据库对物质进行定性,包括质谱匹配及保留时间指数匹配。最后去除QC样本中检出率50%以下或RSD>30%的峰。
1.3 统计分析生长指标数据结果均采用平均值±标准误表示。每个代谢物的离子峰响应值与内标响应值进行比较得到相对含量,导入SPSS16.0软件进行主成分分析(PCA),通过载荷图直观地表达组间的差异,挖掘一些分类贡献率最大的代谢物。再用MeV软件制作热图,由聚类分析可以获得不同样品或化合物的归类现象。利用单因素方差分析(LSD)显著性统计检验(P < 0.001)挖掘高度显著的差异代谢物。
2 结果和分析 2.1 硒对中华羊茅地理种群共生体和非共生体生长指标的影响硒对青海中华羊茅2个地理种群(HB和YS)农艺性状的影响结果见表 1。株高和根长在中华羊茅地理种群、E+和E–植株及硒处理之间均差异不明显(P>0.05)。而地理种群HB经硒处理后E+植株中分蘖数高于不添加Se的地理种群YS E+和加硒的E–植株(P<0.05),但同种地理种群E+和E–植株中,硒处理与对照之间分蘖数不存在显著性差异(P>0.05)。中华羊茅地理种群E+和E–植株根系形态如图 1,根直径不存在明显差异(P>0.05)。
| Growth index | Geographical populations and Se treatment | |||||||
| CKHBE+ | SeHBE+ | CKHBE– | SeHBE– | CKYSE+ | SeYSE+ | CKYSE– | SeYSE– | |
| Plant heigh/cm | 41.83±1.89a | 42.23±1.66a | 39.73±1.93a | 40.20±1.55a | 40.83±1.44a | 39.53±1.55a | 39.53±2.53a | 39.67±1.72a |
| Tiller number (plant) | 4.33±0.58ab | 4.67±0.58a | 4.33±0.58ab | 3.33±0.58bc | 3.00±1.00c | 3.33±0.58bc | 3.00±1.00c | 3.67±0.58abc |
| Root length/cm | 12.63±1.19a | 12.77±1.12a | 11.23±0.25a | 11.33±0.58a | 11.43±0.60a | 11.47±0.84a | 11.37±1.85a | 11.63±1.21a |
| Non-matching lower case letters in the same line indicated a significant effect of selenium treatment (P < 0.05). CKHBE+ and CKHBE– respectively represented HB with and without Epichloë endophyte under control; SeHBE+ and SeHBE– respectively represented HB with and without Epichloë endophyte under Se treatment; CKYSE+ and CKYSE– respectively represented YS with and without Epichloë endophyte under control; SeYSE+ and SeYSE– respectively represented YS with and without Epichloë endophyte under Se treatment. | ||||||||

|
| 图 1 中华羊茅地理种群HB和YS根系 Figure 1 Roots of F. sinensis geographical populations HB and YS. CKRHBE+, SeRHBE+, CKRHBE–, SeRHBE–, CKRYSE+, SeRYSE+, CKRYSE– and SeRYSE– were arranged from left to right; CKRHBE+ and CKRHBE– represented HB roots with and without Epichloë endophyte under control, respectively; SeRHBE+ and SeRHBE– represented HB roots with and without Epichloë endophyte under Se treatment, respectively; CKRYSE+ and CKRYSE– represented YS roots with and without Epichloë endophyte under control, respectively; SeRYSE+ and SeRYSE– represented YS roots with and without Epichloë endophyte under Se treatment, respectively. |
2.2 硒对中华羊茅两个地理种群的E+、E–植株代谢物的影响
从植物样品中共检测到484个峰,与数据库比对,分别从中华羊茅地理种群HB和YS植株中鉴定出的206种和205种化合物。
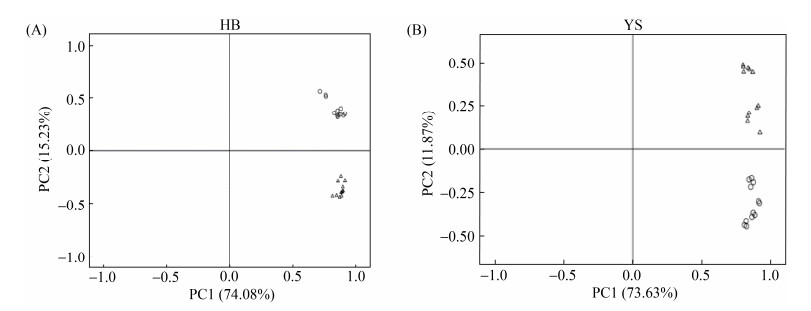
2.2.1 中华羊茅地理种群HB和YS植株地上部分和根系的主成分分析:利用PCA方法,对中华羊茅地理种群HB带菌情况(E+和E–)、部位(地上部分和根系部分)及硒处理共8个样品(每个样品3个重复数)进行处理,提取出2个主成分如图 2所示(图 2-A),第一主成分所占有的分量为74.08%,第二主成分占15.23%,基本能反应处理的最大特征。样品明显地分为两类:根系样品和叶片样品,说明不同部位代谢产物的表达存在差异。对植物YS带菌情况(E+和E–)、部位(地上部分和根系部分)及硒处理共8个样品(每个样品3个重复数)进行PCA分析,提取出2个主成分结果如图 2-B,第一主成分和第二主成分所占有的分量分别为73.63%和11.87%。地理种群YS样品分为根系样品和叶片样品两类,表明两者之间的代谢产物存在差异性,根系样品中E+和E–之间也存在差异性,叶片样品中对照与硒处理之间具有差异性。
|
| 图 2 中华羊茅地理种群HB和YS地上部分和根系样品主成分分析(△和○分别代表地上部分和根系样品) Figure 2 Principal component analysis (PCA) of the shoot and root samples for F. sinensis geographical populations HB and YS. △ and ○ represented shoot and root samples, respectively. |
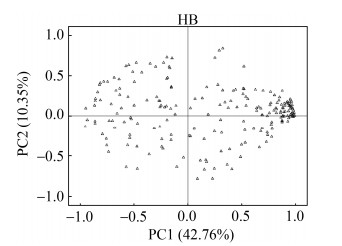
对地理种群HB已确定的206种化合物进行主成分分析,提取出22个主成分,其中前2个主成分累计贡献率为53.10% (图 3)。在第一主成分上载荷较大的有methylmalonic acid、maleic acid、serine、2, 3-dimethylsuccinic acid、citramalic acid、L-glutamic acid、phenylalanine、(2R, 3S)-2- hydroxy-3-isopropylbutanedioic acid、D-alanyl- D-alanine、D-erythronolactone、glutamic acid、ribose、D-arabitol、ribitol、diglycerol、glucose-1- phosphate、2-deoxy-D-glucose、shikimic acid、glucuronic acid、sedoheptulose、4-hydroxycinnamic acid、caffeic acid、galactonic acid、isopropyl-β- D-thiogalactopyranoside、β-mannosylglycerate、D-erythro-sphingosine、DL-dihydrosphingosine、2-monopalmitin、galactinol、chlorogenic acid、α-tocopherol、raffinose等32种化合物。
|
| 图 3 中华羊茅地理种群HB的206种化合物主成分分析 Figure 3 Principal component analysis (PCA) of the 206 metabolites in F. sinensis geographical population HB. |
而对地理种群YS已确定的205种化合物进行主成分分析,提取出20个主成分,其中前2个主成分累计贡献率为51.51% (图 4)。在第一主成分上载荷较大的有citramalic acid、ribose、diglycerol、dehychroshikimic acid、glucose-1-phosphate、glucuronic acid、sedoheptulose、ascorbate、tyrosine、caffeic acid、galactonic acid、isopropyl-β- D-thiogalactopyranoside、3, 4-dihydrosphingosine、D-erythro-sphingosine、DL-dihydrosphingosine、5-dihydrocortisol,第二主成分上载荷较大的有2-deoxyuridine、N-cyclohexylformamide、4-acetylbutyric acid、maleamate、N-formyl-L- methionine,仅β-alanine在第三主成分载荷较大。

|
| 图 4 中华羊茅地理种群YS的205种化合物主成分分析 Figure 4 Principal component analysis (PCA) of the 205 metabolites in F. sinensis geographical population YS. |
2.2.2 中华羊茅地理种群HB和YS植株地上部分和根系的聚类分析:
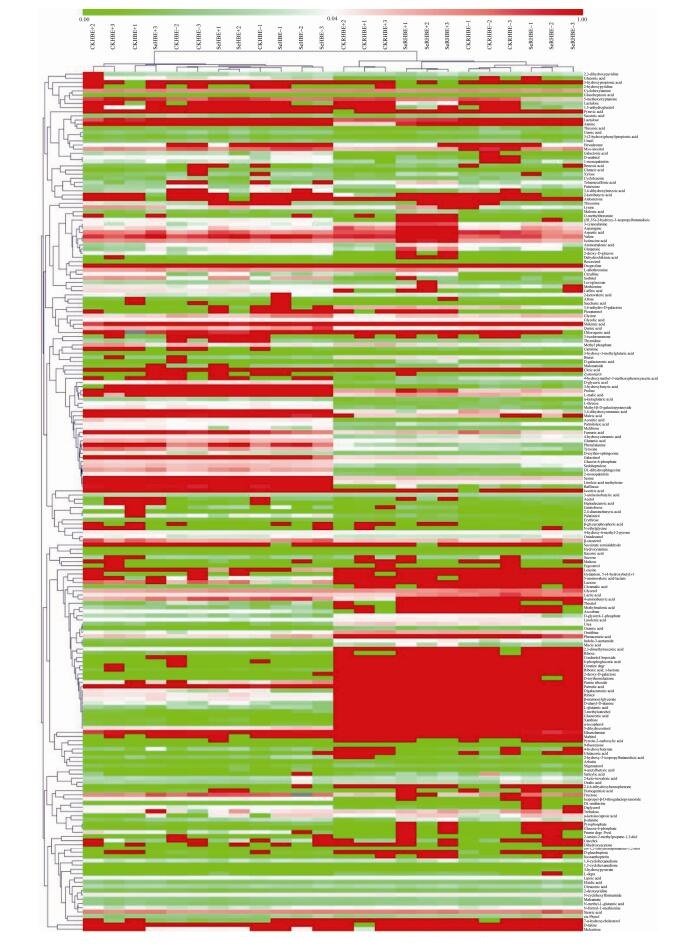
地理种群HB聚类分析分为两大类(图 5):一大类包括113种化合物,其中又分成31种和82种(分为50种和32种);另一大类有93种化合物,其中又分为39种和54种。样品聚类情况与PCA结果相同,分为叶片样品和根系样品两类。
|
| 图 5 中华羊茅地理种群HB代谢水平热图 Figure 5 Heatmap of metabolite levels in F. sinensis geographical population HB. CKHBE+ and CKHBE– represented HB shoots with and without Epichloë endophyte under control, respectively; SeHBE+ and SeHBE– represented HB shoots with and without Epichloë endophyte under Se treatment respectively; CKRHBE+ and CKRHBE– represented HB roots with and without Epichloë endophyte under control, respectively; SeRHBE+ and SeRHBE– represented HB roots with and without Epichloë endophyte under Se treatment respectively; 1, 2 and 3 represented the repetition number of sample. |
地理种群YS聚类分析分为三类(图 6):5种化合物聚为一类,9种化合物聚为另一类,还有192种聚成一类,其中又分成40种、90种、48种及14种4类。样品聚类情况与PCA结果相同,分为叶片样品和根系样品两大类。

|
| 图 6 中华羊茅地理种群YS代谢水平热图 Figure 6 Heatmap of metabolite levels in F. sinensis geographical population YS. CKYSE+ and CKYSE– represented YS shoots with and without Epichloë endophyte under control, respectively; SeYSE+ and SeYSE– represented YS shoots with and without Epichloë endophyte under Se treatment, respectively; CKRYSE+ and CKRYSE– represented YS roots with and without Epichloë endophyte under control, respectively; SeRYSE+ and SeRYSE– represented YS roots with and without Epichloë endophyte under Se treatment, respectively; 1, 2 and 3 represented the repetition number of sample. |
2.2.3 中华羊茅地理种群HB和YS地上部分和根系的代谢产物变化情况:
中华羊茅地理种群HB代谢产物比值结果见表 2。共生体(E+)与非共生体(E–)相比较,对照和硒处理后地上部分中化合物相对含量高的所占百分比分别为57.77%和49.03%,此化合物含量高的百分比高于化合物含量低的百分比;而根系中化合物相对含量低的占比高于化合物相对含量高的百分比。与对照相比,施加亚硒酸钠提高E+和E–地上部分代谢产物含量;亚硒酸钠对E+和E–根系影响不一致,硒促进E+植株中根系产生含量高的代谢物,相反,降低E–植株中根系代谢产物的含量。相同处理下地上部分代谢产物与根系代谢产物之比值大于1的百分比高于两者之比值小于1的百分比,说明地上部分积累的代谢产物含量较高。
| Ratio | Percentage of ratio above 1/% | Percentage of ratio below 1/% | Percentage of undetect compound/% |
| CKHBE+/CKHBE– | 57.77 | 33.98 | 8.25 |
| CKRHBE+/CKRHBE– | 37.86 | 43.69 | 8.45 |
| SeHBE+/SeHBE– | 49.03 | 44.66 | 6.31 |
| SeRHBE+/SeRHBE– | 39.81 | 42.23 | 17.96 |
| SeHBE+/CKHBE+ | 47.57 | 47.09 | 5.34 |
| SeHBE–/CKHBE– | 56.31 | 35.44 | 8.25 |
| SeRHBE+/CKRHBE+ | 47.09 | 34.95 | 17.96 |
| SeRHBE–/CKRHBE– | 32.04 | 49.51 | 15.45 |
| CKHBE+/CKRHBE+ | 45.63 | 34.47 | 19.90 |
| SeHBE+/SeRHBE+ | 42.72 | 37.38 | 19.90 |
| CKHBE–/CKRHBE– | 41.75 | 39.81 | 8.45 |
| SeHBE–/SeRHBE– | 45.63 | 35.92 | 18.45 |
| CKHBE+ and CKHBE– represented HB shoots with and without Epichloë endophyte under control, respectively; SeHBE+ and SeHBE– represented HB shoots with and without Epichloë endophyte under Se treatment, respectively; CKRHBE+ and CKRHBE– represented HB roots with and without Epichloë endophyte under control, respectively; SeRHBE+ and SeRHBE– represented HB roots with and without Epichloë endophyte under Se treatment, respectively. | |||
从表 3中华羊茅地理种群YS代谢产物比值可知,E+与E–植株相比较,对照地上部分和对照根系中化合物相对含量高的所占百分比分别为46.34%和53.66%,高于化合物含量低的百分比;而施加硒地上部分和根系化合物相对含量高的占比少于化合物相对含量高的百分比。与不加硒相比,施加亚硒酸钠提高E–地上部分和根系代谢产物含量;相反,亚硒酸钠降低E+地上部分和根系代谢产物的含量。相同处理下地上部分代谢产物与根系代谢产物之比值大于1的百分比高于两者之比值小于1的百分比,说明地上部分积累更多的代谢产物。
| Ratio | Percentage of ratio above 1/% | Percentage of ratio below 1/% | Percentage of undetect compound/% |
| CKYSE+/CKYSE– | 46.34 | 43.41 | 10.24 |
| CKRYSE+/CKRYSE– | 53.66 | 21.95 | 24.39 |
| SeYSE+/SeYSE– | 24.39 | 66.34 | 14.15 |
| SeRYSE+/SeRYSE– | 21.46 | 59.02 | 19.51 |
| SeYSE+/CKYSE+ | 37.56 | 51.71 | 10.73 |
| SeYSE–/CKYSE– | 51.71 | 38.05 | 10.24 |
| SeRYSE+/CKRYSE+ | 32.68 | 48.29 | 19.02 |
| SeRYSE–/CKRYSE– | 52.68 | 22.93 | 24.39 |
| CKYSE+/CKRHBE+ | 41.95 | 39.02 | 19.02 |
| SeYSE+/SeRHBE+ | 43.90 | 36.59 | 19.51 |
| CKYSE–/CKRYSE– | 40.98 | 35.13 | 23.90 |
| SeYSE–/SeRYSE– | 47.80 | 32.20 | 20.00 |
| CKYSE+ and CKYSE– represented YS shoots with and without Epichloë endophyte under control, respectively; SeYSE+ and SeYSE– represented YS shoots with and without Epichloë endophyte under Se treatment, respectively; CKRYSE+ and CKRYSE– represented YS roots with and without Epichloë endophyte under control, respectively; SeRYSE+ and SeRYSE– represented YS roots with and without Epichloë endophyte under Se treatment, respectively. | |||
2.2.4 中华羊茅地理种群HB和YS地上部分和根系的显著性分析:
分别对地理种群HB地上部分或根系206种化合物在处理间进行了各个代谢物相对含量的显著性分析,发现地上部分82种化合物相对含量在处理之间存在显著(P < 0.05)或极显著(P < 0.01)差异,根据高度显著P < 0.001值来筛选差异代谢物,得出有27种差异代谢物(图 7);根系有99种代谢产物相对含量在处理之间存在显著(P < 0.05)或极显著(P < 0.01)差异,根据高度显著P < 0.001值来筛选差异代谢物,得出有42种差异代谢物(图 8)。就地上部分27种差异代谢物,其中地理种群HB E+植株在施加硒或不加硒条件下有12种化合物(methionine、lysine、tyrosine、citruline、maleic acid、fumaric acid、ribitol、sorbitol、4-hydroxycinnamic acid、caffeic acid、xanthine、isopropyl-β-D-thiogalactopyranoside)含量极显著高于施加硒或不加硒的E–植株(P < 0.001);相反,有3种代谢物(glycine、cycloleucine、malonamide)在E+植株中含量极显著低于E–植株(P < 0.001)。硒处理的E+和E–植株地上部分有3种化合物(α-ketoisocaproic acid、carnitine、purine riboside)极显著高于对照E+和E–植株(P < 0.001),而仅化合物methyl phosphate含量在对照E+和E–植株中极显著多于加硒E+和E–植株(P < 0.001)。施加硒E+植株地上部分有3种化合物aspartic acid、DL-dihydrosphingosine、2-monopalmitin丰度极显著超过施加硒的E–和对照组植株(P < 0.001),有3种化合物β-alanine、chlorogenic acid、5-dihydrocortisol在加硒处理的E–植株中含量极显著高于加硒E+和对照组植株(P < 0.001)。

|
| 图 7 地理种群HB地上部分差异代谢物 Figure 7 Marked metabolites from shoots of geographical population HB. CKHBE+ and CKHBE– represented HB shoots with and without Epichloë endophyte under control, respectively; SeHBE+ and SeHBE– represented HB shoots with and without Epichloë endophyte under Se treatment, respectively. |
|
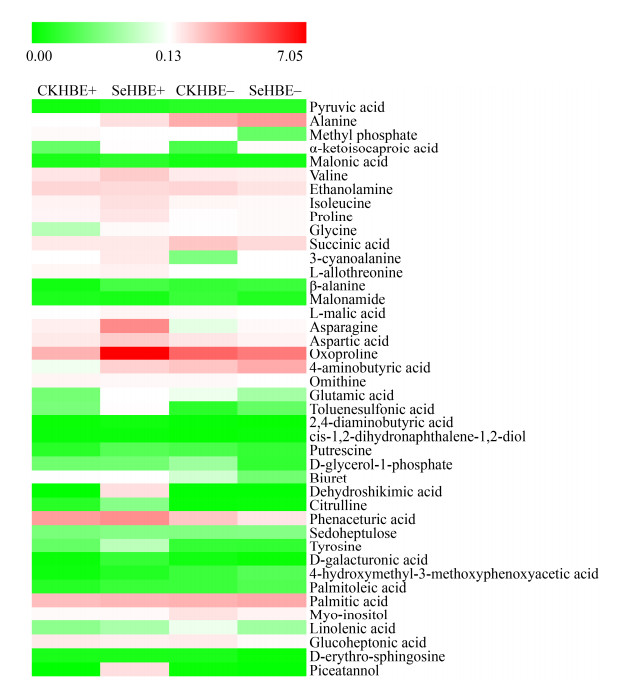
| 图 8 地理种群HB根系差异代谢物 Figure 8 Marked metabolites from roots of geographical population HB. CKRHBE+ and CKRHBE– represented HB roots with and without Epichloë endophyte under control, respectively; SeRHBE+ and SeRHBE– represented HB roots with and without Epichloë endophyte under Se treatment, respectively. |
HB根系42种差异代谢物变化情况受植株带菌、加硒条件的作用。E+植株在加硒和对照条件下根系中有5种化合物(asparagine、tyrosine、L-allothreonine、biuret、phenaceturic acid)含量极显著大于加硒和对照的E–植株(P < 0.001),但有7种化合物含量(alanine、4-aminobutyric acid、pyruvic acid、succinic acid、dehydroshikimic acid、piceatannol、4-hydroxymethyl-3-methoxyphenoxyacetic acid)在加硒和对照条件下E–植株根系中极显著多于在加硒和对照条件下E+植株根系(P < 0.001)。加硒促进E+和E–植株极显著地积累glycine、α-ketoisocaproic acid化合物水平(P < 0.001);相反,不加硒的E+和E–植株极显著地积累glucoheptonic acid和ethanolamine含量(P < 0.001)。E+植株根系在施加硒后有(valine、isoleucine、proline、β-alanine、aspartic acid、citrulline、oxoproline、3-cyanoalanine、malonic acid、D-galacturonic acid、L-malic acid、2, 4-diaminobutyric acid、toluenesulfonic acid) 13种化合物含量极显著超过其他处理(P < 0.001),E+植株加硒后glutamic acid和putrescine化合物含量极显著超过对照E+植株和硒处理E–植株(P < 0.001)。在E–植株中加硒处理的根系产生palmitoleic acid和palmitic acid含量极显著超过其他组(P < 0.001)。
分别对地理种群YS地上部分或根系205种化合物在处理间进行了各个代谢物相对含量的显著性分析,发现地上部分115种化合物相对含量在处理之间存在显著(P < 0.05)或极显著(P < 0.01)差异,根据极显著P < 0.001值来筛选差异代谢物,得出有50种差异代谢物(图 9);根系有81种代谢产物相对含量在处理之间存在显著(P < 0.05)或极显著(P < 0.01)差异,根据极显著P < 0.001值来筛选差异代谢物,得出有33种差异代谢物(图 10)。
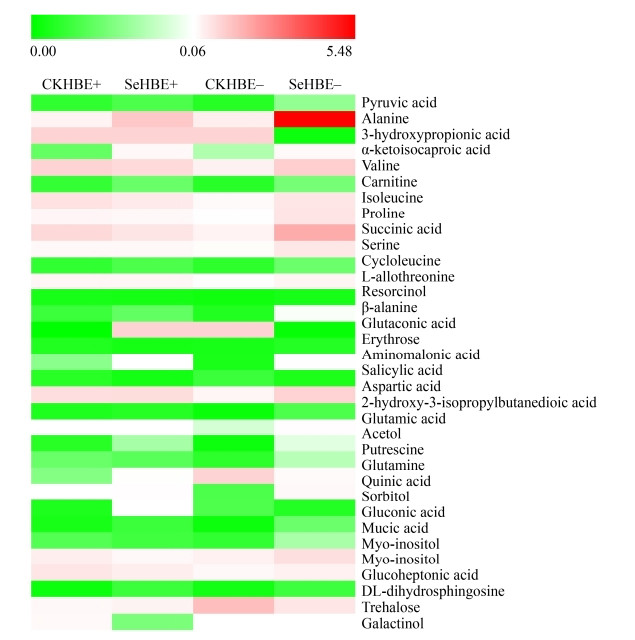
|
| 图 9 地理种群YS地上部分差异代谢物 Figure 9 Marked metabolites from shoots of geographical population YS. CKYSE+ and CKYSE– represented YS shoots with and without Epichloë endophyte under control, respectively; SeYSE+ and SeYSE– represented YS shoots with and without Epichloë endophyte under Se treatment, respectively. |

|
| 图 10 地理种群YS根系差异代谢物 Figure 10 Marked metabolites from roots of geographical population YS. CKRYSE+ and CKRYSE– represented YS roots with and without Epichloë endophyte under control, respectively; SeRYSE+ and SeRYSE– represented YS roots with and without Epichloë endophyte under Se treatment, respectively. |
地理种群YS地上部分40种差异代谢物变化与植物带菌、硒处理有关。E+植株在加硒和对照条件下地上部分中有2种化合物3-cyanoalanine、2-deoxy-D-glucose极显著低于加硒和对照的E–植株(P < 0.001),而化合物2, 3-dimethylsuccinic acid在加硒和对照E+植株中含量极显著高于加硒和对照的E–植株(P < 0.001)。加硒促进E+和E–植株极显著地积累4种化合物的含量(α-ketoisocaproic acid、citric acid、acetol、purine riboside) (P < 0.001);但对照E+和E–植株有9种化合物含量(glycine、serine、ethanolamine、3-hydroxypropionic acid、raffinose、glycerol、methyl-β-D-galactopyranoside、phenaceturic acid、mucic acid)极显著多于加硒组(P < 0.001)。YS地上部分E–植株在施加硒后有14种化合物(3-aminoisobutyric acid、4-aminobutyric acid、ribose、D-talose、α-tocopherol、erythrose、N-methyl-L- glutamic acid、octadecanol、3-methylcatechol、2-hydroxypyridine、2, 3-dihydroxypyridine、1, 3-cyclohexanedione、4-hydroxy-6-methyl-2-pyrone、cis-1, 2-Dihydronaphthalene-1, 2-diol)极显著高于其他组(P < 0.001),化合物aconitic acid在加硒的E–植株的含量极显著超过加硒E+植株和对照E–植株(P < 0.001),加硒E+植株中化合物ribitol和4-hydroxycinnamic acid含量极显著超过其他组(P < 0.001)。
植物带菌和硒处理影响着地理种群YS根系33种差异代谢物的变化。加硒促进E+和E–植株极显著地积累9种化合物(alanine、β-alanine、cycloleucine、pyruvic acid、α-ketoisocaproic acid、carnitine、DL-dihydrosphingosine、acetol、gluconic acid) (P < 0.001),但对照E+和E–植株仅salicylic acid化合物含量极显著高于加硒组(P < 0.001)。YS E–植株根系在施加硒后有15种化合物(valine、proline、serine、glutamine、2-hydroxy-3-isopropyl、butanedioic acid、glutamic acid、putrescine、aminomalonic acid、3-hydroxypropionic acid、succinic acid、quinic acid、mucic acid、glutaconic acid、myo-inositol)极显著超过其他组(P < 0.001),化合物sorbitol在加硒的E+植株含量极显著高于其他组,化合物erythrose在加硒的E–植株的含量极显著多于加硒E+和对照E–植株(P < 0.001),E–植株中化合物trehalose含量极显著大于对照E+和加硒E+植株。
2.2.5 比较中华羊茅地理种群HB和YS植株地上部分和根系的差异性代谢物:从图 7和图 8可知HB植株地上部分和根系共同差异代谢物有9种:methyl phosphate、α-ketoisocaproic acid、glycine、β-alanine、malonamide、aspartic acid、putrescine、citrulline、tyrosine。图 9和图 10表明YS植株地上部分和根系具有8种共同差异代谢物:3-hydroxypropionic acid、α-ketoisocaproic acid、proline、serine、erythrose、aminomalonic acid、acetol、mucic acid。
根据图 7 HB植株地上部分和图 9 YS植株地上部分差异代谢物,发现两者共同的差异代谢物有5种:α-ketoisocaproic acid、glycine、malonamide、ribitol、purine riboside。由图 8和图 10可知,HB植株根系和YS植株根系共同差异代谢物有14种:pyruvic acid、alanine、α-ketoisocaproic acid、valine、isoleucine、proline、succinic acid、L-allothreonine、β-alanine、aspartic acid、glutamic acid、putrescine、myo-inositol、glucoheptonic acid。
3 讨论和结论中华羊茅的生长状况受带菌情况、矿质成分处理以及地理种群等多方面的影响[27, 32]。王凯等[27]在温室中种植来源于青海、四川的6个中华羊茅地理种群,通过测量发现内生真菌对部分地理种群中华羊茅的株高、分蘖影响不一致。王美宁等[32]分别采用ZnCl2 (500 mg/L)或CdCl2 (100 mg/L)处理中华羊茅E+和E–植株,结果表明,与对照相比施加Zn能显著增加E+和E–植株的株高,而添加Cd显著抑制其生长(P < 0.05);两种处理对中华羊茅分蘖能力作用有差别,Zn处理对中华羊茅E+和E–植株分蘖数的影响不明显;Cd处理明显促进E+产生分蘖数,但抑制E–植株分蘖能力(P < 0.05)。本试验发现硒处理对2个地理种群E+和E–植株株高、根长、根直径均没有显著影响;地理种群HB经硒处理后E+植株中分蘖数高于硒处理E–植株、不添加Se的YS E+和E–植株(P < 0.05)。
Lee等[35]研究表明,添加不同浓度的硒酸钠使花椰菜总氨基酸和谷氨酰胺得到提高;Tian等[2]采用GC-MS技术检测花椰菜应答硒(100 μmol/L)的代谢产物,发现施加硒酸钠促进serine、D-erythronolactone、melezitose、tyrosine、glucose- 6-phosphate、β-alanine、S-carboxymethylcysteine、D-fructose 1, 6-bisphosphate含量上升,而降低D-glyceric acid、succinic acid、citric acid、L-malic acid、D-talose、threonine、O-acetylserine的含量。Hoewyk等[36]从转录组学方面研究拟南芥响应硒胁迫后根系和地上部分基因表达差异,发现340个基因表达量明显下调,而有553个基因表达量增加,这些上调的基因掺入信号转导(钙信号、乙烯、茉莉酸)、转录因子、调控蛋白、胁迫相关蛋白(热激蛋白)、硫吸收与转运、氨基酸合成、碳水化合物合成等多种代谢途径。本研究结果表明差异性代谢物变化受中华羊茅地理种群、带菌情况、组织部位、加硒条件等因素影响。与不带菌植株相比,硒处理提高了地理种群HB带菌植株地上部分中多种非靶标代谢产物的相对含量(表 2)。施加硒促使2个地理种群(HB和YS)地上部分和根系中某些差异性代谢物发生极显著的上升或降低变化。HB经硒处理后E+和E–植株地上部分中α-ketoisocaproic acid、carnitine、purine riboside的含量极显著上升(P < 0.001),而methyl phosphate的含量极显著降低(P < 0.001);E+和E–植株根系中glycine和α-ketoisocaproic acid含量极显著积累(P < 0.001),而glucoheptonic acid和ethanolamine含量极显著减少(P < 0.001)。硒处理有利于YS E+和E–植株地上部分α-ketoisocaproic acid、citric acid、acetol和purine riboside的累积(P < 0.001),但不利于9种化合物的累积(glycine、serine、ethanolamine、3-hydroxypropionic acid、raffinose、glycerol、methyl-β-D-galactopyranoside、phenaceturic acid、mucic acid) (P < 0.001);YS E+和E–植株根系中有9种化合物含量极显著增加(alanine、β-alanine、cycloleucine、pyruvic acid、α-ketoisocaproic acid、carnitine、DL-dihydrosphingosine、acetol、gluconic acid,P < 0.001),仅salicylic acid含量受到极显著抑制(P < 0.001)。
地理种群和内生真菌影响着中华羊茅植株的营养成分。通过研究6个地理种群中华羊茅带菌植株与不带菌植株的粗蛋白和粗脂肪含量,发现2个地理种群E+植株粗脂肪和粗蛋白含量均显著多于E–植株(P < 0.05);3个地理种群带菌植株粗脂肪含量显著高于不带菌植株(P < 0.05),其他3个地理种群带菌植株粗脂肪与不带菌植株无显著差异(P > 0.05);3个地理种群E+植株粗蛋白含量明显超过E–植株(P < 0.05),而2个地理种群E+植株粗蛋白明显低于E–植株(P < 0.05)[27]。基于代谢组学对禾草-内生真菌共生体互作关系的研究,证明了内生真菌能改变植物体内含氮化合物、碳水化合物、有机酸和脂肪酸等代谢产物的水平[16-17]。本研究发现内生真菌提高地理种群HB和YS植株地上部分中多种非靶标代谢产物的相对含量(表 2,表 3)对2个地理种群地上部分和根系差异性代谢物变化有高度显著影响。在硒处理或对照条件下带菌HB植株地上部分有12种化合物(methionine、lysine、tyrosine、citruline、maleic acid、fumaric acid、ribitol、sorbitol、4-hydroxycinnamic acid、caffeic acid、isopropyl-β- D-thiogalactopyranoside、xanthine)的含量极显著高于不带菌植株(P < 0.001),但带菌植株有3种代谢物(glycine、cycloleucine、malonamide)的含量极显著低于不带菌植株(P < 0.001);带菌植株根系有5种化合物(asparagine、tyrosine、L-allothreonine、biuret、phenaceturic acid)的含量极显著高于E–植株(P < 0.001),然而带菌植株根系7种化合物(alanine、4-aminobutyric acid、pyruvic acid、succinic acid、dehydroshikimic acid、piceatannol、4-hydroxymethyl-3-methoxyphenoxyacetic acid)的含量极显著低于不带菌植株(P < 0.001)。内生真菌对YS植株的差异代谢物影响与HB不一致,与E–植株相比,在加硒和对照条件下带菌植株根系中3-cyanoalanine和2-deoxy-D-glucose含量极显著增加(P < 0.001),而2, 3-dimethylsuccinic acid的含量极显著降低(P < 0.001)。
综上所述,中华羊茅2个地理种群应答硒处理后地上部分和根系非靶标代谢产物和差异性代谢物存在一定的差异性,内生真菌和/或硒处理能提高地上部分一些非靶标代谢产物或差异性代谢物含量,以此来改良中华羊茅牧草品质,为开发富硒牧草提供科学依据。
| [1] | Li ZY, Guo SY, Li L. Bioeffects of selenite on the growth of Spirulina platensis and its biotransformation. Bioresource Technology, 2003, 89(2): 171-176. DOI:10.1016/S0960-8524(03)00041-5 |
| [2] | Tian M, Xu XY, Liu FX, Fan X, Pan SY. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food Research International, 2018, 111: 205-211. DOI:10.1016/j.foodres.2018.04.020 |
| [3] | Yasin M, El-Mehdawi AF, Pilon-Smits EAH, Faisal M. Selenium-fortified wheat: potential of microbes for biofortification of selenium and other essential nutrients. International Journal of Phytoremediation, 2015, 17(8): 777-786. DOI:10.1080/15226514.2014.987372 |
| [4] | Durán P, Acuña JJ, Gianfreda L, Azcón R, Funes-Collado V, Mora ML. Endophytic selenobacteria as new inocula for selenium biofortifcation. Applied Soil Ecology, 2015, 96: 319-326. DOI:10.1016/j.apsoil.2015.08.016 |
| [5] | Tognon GB, Sanmartín C, Alcolea V, Cuquel FL, Goicoechea N. Mycorrhizal inoculation and/or selenium application affect postharvest performance of snapdragon flowers. Plant Growth Regulation, 2016, 78: 389-400. DOI:10.1007/s10725-015-0100-8 |
| [6] |
Qiao YJ, Gao Y, Lan DD, Liang J, Li JH. Effect of AM fungi and selenium content on seeding quality of Salvia miltiorrhiza Bge. Medical Research and Education, 2015, 32(2): 1-5.
(in Chinese) 乔亚君, 高颖, 兰丹丹, 梁洁, 李建恒. 接种AM真菌和喷施硒对丹参幼苗的影响. 医学研究与教育, 2015, 32(2): 1-5. DOI:10.3969/j.issn.1674-490X.2015.02.001 |
| [7] | Christensen MJ, Leuchtmann A, Rowan DD, Tapper BA. Taxonomy of Acremonium endophytes of tall fescue (Festuca arundinacea), meadow fescue (Festuca pratensis) and perennial ryegrass (Lolium perenne). Mycological Research, 1993, 97(9): 1083-1092. DOI:10.1016/S0953-7562(09)80509-1 |
| [8] | Christensen MJ, Bennett RJ, Schmid J. Growth of Epichloё/Neotyphodium and p-endophytes in leaves of Lolium and Festuca grasses. Mycological Research, 2002, 106(1): 93-106. DOI:10.1017/S095375620100510X |
| [9] | Leuchtmann A, Bacon CW, Schardl CL, White JF, Tadych M. Nomenclatural realignment of Neotyphodium species with genus Epichloё. Mycologia, 2014, 106(2): 202-215. DOI:10.3852/13-251 |
| [10] | Johnson LJ, de Bonth ACM, Briggs LR, Caradus JR, Finch SC, Fleetwood DJ, Fletcher LR, Hume DE, Johnson RD, Popay AJ, Tapper BA, Simpson WR, Voisey CR, Card SD. The exploitation of epichloae endophytes for agricultural benefit. Fungal Diversity, 2013, 60(1): 171-188. DOI:10.1007/s13225-013-0239-4 |
| [11] | Song ML, Chai Q, Li XZ, Yao X, Li CJ, Christensen MJ, Nan ZB. An asexual Epichloë endophyte modifies the nutrient stoichiometry of wild barley (Hordeum brevisubulatum) under salt stress. Plant Soil, 2015, 387(1/2): 153-165. |
| [12] | Song ML, Li XZ, Saikkonen K, Li CJ, Nan ZB. An asexual Epichloë endophyte enhances waterlogging tolerance of Hordeum brevisubulatum. Fungal Ecology, 2015, 13: 44-52. DOI:10.1016/j.funeco.2014.07.004 |
| [13] | Hesse U, Schöberlein W, Wittenmayer L, Förster K, Warnstorff K, Diepenbrock W, Merbach W. Effects of Neotyphodium endophytes on growth, reproduction and drought-stress tolerance of three Lolium perenne L. genotypes. Grass and Forage Science, 2003, 58(4): 407-415. DOI:10.1111/j.1365-2494.2003.00393.x |
| [14] | Tian P, Nan ZB, Li CJ, Spangenberg G. Effect of the endophyte Neotyphodium lolii on susceptibility and host physiological response of perennial ryegrass to fungal pathogens. European Journal of Plant Pathology, 2008, 122(4): 593-602. DOI:10.1007/s10658-008-9329-7 |
| [15] | Vignale MV, Novas MV, Astiz-Gassó MM, Iannone LJ. Epichloë endophytes confer resistance to the smut Ustilago bullata in the wild grass Bromus auleticus (Trin.). Biological Contro, 2013, 67(1): 1-7. DOI:10.1016/j.biocontrol.2013.06.002 |
| [16] | Rasmussen S, Parsons AJ, Fraser K, Xue H, Newman JA. Metabolic profiles of Lolium perenne are differentially affected by nitrogen supply, carbohydrate content, and fungal endophyte infection. Plant Physiology, 2008, 146(3): 1440-1453. DOI:10.1104/pp.107.111898 |
| [17] | Nagabhyru P, Dinkins RD, Wood CL, Bacon CW, Schardl CL. Tall fescue endophyte effects on tolerance to water-deficit stress. BMC Plant Biology, 2013, 13(1): 127. DOI:10.1186/1471-2229-13-127 |
| [18] |
Jin WJ, Li CJ, Nan ZB. Biological and physiological characteristics of Neotyphodium endophyte symbiotic with Festuca sinensis. Mycosystema, 2009, 28(3): 363-369.
(in Chinese) 金文进, 李春杰, 南志标. 中华羊茅内生真菌Neotyphodium sp.生物学与生理学特性的研究. 菌物学报, 2009, 28(3): 363-369. |
| [19] |
Yang Y, Chen N, Li CJ. The morphological diversity of endophytic fungus in Festuca sinensis in Gansu Province. Pratacultural Science, 2011, 28(2): 273-278.
(in Chinese) 杨洋, 陈娜, 李春杰. 甘肃中华羊茅内生真菌形态多样性. 草业科学, 2011, 28(2): 273-278. DOI:10.3969/j.issn.1001-0629.2011.02.020 |
| [20] |
Huang Y, Wang JJ, Xu WB, Tian P. Analysis of actin sequences from Epichloë endophyte in Festuca sinensis. Acta Prataculturae Sinica, 2016, 25(9): 125-131.
(in Chinese) 旷宇, 汪建军, 许文博, 田沛. 中华羊茅Epichloë内生真菌的actin序列分析. 草业学报, 2016, 25(9): 125-131. |
| [21] | Tian P, Xu WB, Li CJ, Song H, Wang MN, Schardl CL, Nan ZB. Phylogenetic relationship and taxonomy of a hybrid Epichloë species symbiotic with Festuca sinensis. Mycological Progress, 2020, 19(10): 1069-1081. DOI:10.1007/s11557-020-01618-z |
| [22] |
Zhou LY, Wang WN, Zhong S, Pengmao DJ, Ma XL, Luo QY. Effect of fermentation liquid of Epichloë isolated from Festuca sinensis on physiological characteristics of grass. Northern Horticulture, 2018, 14: 72-76.
(in Chinese) 周连玉, 王文妮, 钟松, 鹏毛德吉, 马学兰, 罗巧玉. 中华羊茅内生真菌发酵液对牧草生理特性的影响. 北方园艺, 2018, 14: 72-76. |
| [23] |
Zhou LY, Zhong S, Duo HM, Qiao F. Antimicrobial activity and composition of volatile substance of Epichloë sp. endophyte isolated from Festuca sinensis. Nature Produce Research and Development, 2019, 31(9): 1543-1551.
(in Chinese) 周连玉, 钟松, 朵红梅, 乔枫. 中华羊茅内生真菌Epichloë sp.挥发性物质的抑菌活性及成分分析. 天然产物研究与开发, 2019, 31(9): 1543-1551. |
| [24] | Zhou LY, Zhang XX, Li CJ, Christensen MJ, Nan ZB. Antifungal activity and phytochemical investigation of the asexual endophyte of Epichloë sp. from Festuca sinensis. Science China Life Sciences, 2015, 58(8): 821-826. DOI:10.1007/s11427-015-4845-0 |
| [25] | Tian P, Kuang Y, Lin WH, Wang JJ, Nan ZB. Shoot morphology and alkaloid content of Epichloë endophyte-Festuca sinensis associations. Crop and Pasture Science, 2018, 69(4): 430-438. DOI:10.1071/CP17231 |
| [26] |
Zhou LY, Zhang S, Gengzhou CR, Luo QY. Effect of Epichloë on seeding growth of Festuca sinensis under salt stress. Northern Horticulture, 2016, 9: 65-68.
(in Chinese) 周连玉, 张帅, 更周才让, 罗巧玉. 盐胁迫下内生真菌感染对中华羊茅幼苗生长的影响. 北方园艺, 2016, 9: 65-68. |
| [27] |
Wang K, Wang WL, Wang HB, Pei TY, Dong X, Lin WH, Tian P. Effects of Epichloë endophyte on the growth and nutritional quality of different geographic populations of Festuca sinensis. Pratacultural Science, 2020, 37(3): 522-531.
(in Chinese) 王凯, 王伟林, 王豪邦, 裴天悦, 董鑫, 蔺伟虎, 田沛. 内生真菌对不同地理种群中华羊茅生长及营养品质的影响. 草业科学, 2020, 37(3): 522-531. |
| [28] | Peng QQ, Li CJ, Song ML, Nan ZB. Effects of seed hydropriming on growth of Festuca sinensis infected with Neotyphodium endophyte. Fungal Ecology, 2013, 6(1): 83-91. DOI:10.1016/j.funeco.2012.08.001 |
| [29] | Wang JJ, Zhou YP, Lin WH, Li MM, Wang MN, Wang ZG, Kuang Y, Tian P. Effect of an Epichloë endophyte on adaptability to water stress in Festuca sinensis. Fungal Ecology, 2017, 30: 39-47. DOI:10.1016/j.funeco.2017.08.005 |
| [30] | Zhou LY, Li CJ, Zhang XX, Johnson R, Bao GS, Yao X, Chai Q. Effects of cold shocked Epichloë infected Festuca sinensis on ergot alkaloid accumulation. Fungal Ecology, 2015, 14: 99-104. DOI:10.1016/j.funeco.2014.12.006 |
| [31] | Zhou L, Li C, White JF, Johnson RD. Synergism between calcium nitrate applications and fungal endophytes to increase sugar concentration in Festuca sinensis under cold stress. PeerJ, 2021, 9: e10568. DOI:10.7717/peerj.10568 |
| [32] |
Wang MN, Lin WH, Ma BH, Li MM, Tian P. Effect of endophyte infection on growth and endogenous hormones of Festuca sinensis under Zn and Cd treatments. Pratacultural Science, 2019, 36(9): 2250-2258.
(in Chinese) 王美宁, 蔺伟虎, 马碧花, 李苗苗, 田沛. Zn和Cd处理下内生真菌对中华羊茅生长及内源激素的影响. 草业科学, 2019, 36(9): 2250-2258. |
| [33] |
Yao X, Li XZ, Zhu XX, Li CJ. Effects of two fungicides on Neotyphodium seed-borne fungal endophyte of Festuca sinensis. Pratacultural Science, 2013, 30(10): 1517-1522.
(in Chinese) 姚祥, 李秀璋, 朱小晓, 李春杰. 两种杀菌剂对中华羊茅种传内生真菌的影响. 草业科学, 2013, 30(10): 1517-1522. |
| [34] |
Li CJ, Nan ZB, Liu Y, Paul VH, Dapprich PD. Methodology of endophyte detection of drunken horse grass (Achnatherum inebrians). Edible Fungi of China, 2008, 27(suppl): 105-108.
(in Chinese) 李春杰, 南志标, 刘勇, Paul VH, Dapprich PD. 醉马草内生真菌检测方法的研究. 中国食用菌, 2008, 27(增刊): 105-108. |
| [35] | Lee J, Finley JW, Harnly JM. ffect of selenium fertilizer on free amino acid composition of broccoli (Brassica oleracea Cv. Majestic) determined by gas chromatography with fame ionization and mass selective detection. Journal of Agricultural and Food Chemistry, 2005, 53(23): 9105-9111. DOI:10.1021/jf051221x |
| [36] | Hoewyk DV, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smit EAH. Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiologia Plantarum, 2008, 132(2): 236-253. |
 2021, Vol. 61
2021, Vol. 61




