中国科学院微生物研究所,中国微生物学会
文章信息
- 陈慧贞, 李滢, 谢新强, 张菊梅, 吴清平. 2022
- CHEN Huizhen, LI Ying, XIE Xinqiang, ZHANG Jumei, WU Qingping.
- 益生菌对皮肤光老化的修复作用及其机制研究进展
- Research progress on the mechanism of the repair of skin photoaging by probiotics
- 微生物学报, 62(3): 882-894
- Acta Microbiologica Sinica, 62(3): 882-894
-
文章历史
- 收稿日期:2021-12-03
- 修回日期:2022-01-10
- 网络出版日期:2022-01-20
2. 华南理工大学生物科学与工程学院, 广东 广州 510006
2. School of Biology and Biological Engineering, South China University of Technology, Guangzhou 510006, Guangdong, China
皮肤是人体最大的器官,正常成年人皮肤表面积约1.8 m2,重量约占体重的10%−15%[1]。同时,皮肤也是人体与外界环境之间的第一道屏障,在保护、调节体温、感觉、分泌、排泄和免疫等多方面发挥作用[2]。
当皮肤器官功能出现衰老性损伤、难以适应内外环境变化、发生色泽及弹性下降的情况时,被称为皮肤老化(skin aging)。皮肤老化是人体衰老过程中的一个伴随症状,也是皮肤众多健康问题中最受关注的一个现象。皮肤老化的因素主要分为内因和外因。皮肤衰老的内因主要是遗传因素,当年龄逐渐增大,人体细胞分裂增殖能力下降,皮肤会随之出现变薄、干燥、皱纹细小、出汗不足以及对温度的敏感性增加等老化症状[3]。皮肤衰老的外因主要与紫外辐射和香烟烟雾相关[4–5]。其中,紫外辐射(ultraviolet radiation,UV radiation)导致的皮肤老化又称为光老化(photoaging)[6–7]。光老化是导致皮肤老化的主要外因,80%以上的面部老化均由光老化引起[8]。与遗传所致的皮肤老化特征不同,光老化皮肤表皮层的角质形成细胞活性下降,更新速度减慢,表皮屏障功能减弱,导致皮肤干燥脱皮;真皮层的成纤维细胞数量减少,胶原和弹性蛋白合成减慢而分解加速[9]。光老化皮肤与自然老化皮肤最重要的组织学差异是光老化皮肤真皮组织内无定形弹性纤维呈现过度积累状态,胶原纤维出现明显的异常断裂和结构紊乱[10–11]。老化的皮肤除美观性下降外,其防御损伤的功能也明显受损:受过量UV照射后皮肤屏障的完整性被破坏、分泌功能明显下降,罹患皮肤炎症甚至皮肤恶性肿瘤的风险明显增加[12–13]。
皮肤老化除了与上述生理变化有关之外,还与在皮肤表面的庞大微生态群落系统息息相关[14]。皮肤微生态在防御入侵病原体、调整人体的免疫系统和分解代谢产物方面起着至关重要的作用,因此对皮肤乃至全身健康起到重要作用[15]。在机体衰老过程中,皮肤微生态也随之发生动态变化[16]。作者所在研究团队通过随访长寿家族成员,发现随着年龄的增长,皮肤微生态多样性急剧下降,芽孢杆菌丰度增多,粪球菌丰度减少;皮肤微生物的辅因子及维生素合成、糖原生成及代谢能力逐渐下降,而致癌微生物逐渐增多。Li等发现随着紫外辐射剂量的累积,皮肤微生态也会在不同年龄阶段呈现不同的菌群特征[16]。蓝藻作为儿童组的优势菌群,被证实与防止紫外线损伤和色素沉着有关[17]。Li等推测因为金黄色葡萄球菌、表皮杆菌和乳酸菌能够促进形成成熟而完整的免疫屏障,所以在光老化过程中起到保护作用[16]。不同年龄段的皮肤微生物群落功能预测分析结果显示在光老化过程中最主要的变化是代谢能力的变化、抗氧化能力(辅因子和维生素的代谢)[18]、膜完整性和细胞信号能力(甘油磷脂代谢)[19]、脂质代谢能力(甘油脂代谢、脂肪酸生物合成)[20]和对病原体抵抗能力(抗生素的生物合成)都与光老化程度呈负相关。上述结果表明在皮肤老化进程中皮肤微生态发挥着重要的作用,所以目前国内外对于抗皮肤光老化功能因子的研究从开发植物源性的多肽类、多糖类和黄酮类物质逐渐转向通过微生物手段维持皮肤健康微生态[21]。与此同时,化妆品市场也从植物提取物时代进入微生态护肤时代,通过益生菌调节皮肤微生态的平衡成为科学研究和市场应用的新热点。
益生菌(probiotics)是一类被世界卫生组织定义为通过摄取适当的量、对食用者的身体健康能发挥有效作用的活菌[22]。不同的研究表明,某些特定的益生菌能够积极影响机体微生物区系的组成[23],提升宿主局部器官状态。作者所在研究团队累积了超过两万株的健康功能微生物,建成了相应的菌种资源及基因组数据库并且对其进行功能挖掘,目前已证明益生微生物能通过调节肠道微生态从而在降糖降脂、抗氧化以及抑菌方面有显著的益生功能[24–26]。同样地,大量研究已证实皮肤的微生态系统与皮肤健康状况密切相关,通过益生菌调控皮肤微生态成为促进皮肤健康的新手段[14]。已有研究证实益生菌补充剂对特应性皮炎的治疗有作用[27–29],但在皮肤应用领域中,益生菌并没有被明确定义。虽然在中国化妆品行业国标中要求产品的菌落总数小于等于1 000 CFU/g[30],但在一些欧美国家已经允许含有益生菌活菌成分的产品上架[31],这也印证了益生菌在日化行业广阔的开发前景。本文将从光老化损伤的病理机制及益生菌在皮肤领域的研究现状,系统综述益生菌抑制皮肤光老化研究取得的最新进展。
1 皮肤光老化的机制研究根据波长不同,太阳光中的UV主要有3种类型:长波UV (UVA 320−400 nm)、中波UV (UVB 280−320 nm)和短波UV (UVC 200−280 nm)[32]。UV对皮肤的损害与波长密切相关:短波长UVC具有最强的诱变性,但会被臭氧层阻挡到地球表面;长波长UVA是一种微弱的诱变剂,但它具有很强的穿透能力,可以影响真皮甚至皮下组织区域;中波长UVB的诱变性很强,可以直接导致DNA和RNA损伤从而损伤皮肤细胞[6, 33]。
1.1 紫外辐射对DNA、RNA和蛋白质的直接伤害皮肤抵御UV辐射的第一道防线是通过构象和化学变化吸收UV能量的发色团[34–35]。DNA是表皮中最丰富的发色团,对UV有最强的吸收作用。但过多的UV照射会产生激发电子态和有毒产物,引发DNA损伤[36]。吸收UV时,DNA中的嘧啶碱基与相邻的嘧啶形成键,导致环丁烷-嘧啶二聚体和嘧啶-嘧啶酮(6-4)光产物的形成[37]。这两种光产物可导致功能基因碱基突变,发生复制转录错误,其中含损伤遗传信号的细胞不能得到有序凋亡,严重影响皮肤系统的健康[38]。
除此之外,UV辐照也会损伤细胞内的RNA碱基,导致mRNA不能正常翻译、转录,影响细胞信号的转导[39]。而UV也会导致皮肤细胞内氨基酸发生各种突变,并且影响蛋白质的合成,甚至导致细胞周期停滞和凋亡,这些突变也可以消除细胞的凋亡能力,从而促进皮肤恶性肿瘤的形成[40]。
1.2 紫外辐射对细胞皮肤成分的间接光损伤 1.2.1 活性氧的形成活性氧(reactive oxygen species,ROS)是机体内含氧并且性质活泼的物质的总称,参与细胞损伤和分子信号传递[41]。ROS包括超氧阴离子、羟基自由基和O2衍生的非自由基物种如H2O2等[42]。研究指出,在有氧环境中,被激发的光敏剂可将能量转移到分子氧(O2)上,从而产生ROS[39]。线粒体内产生的ROS约占细胞总ROS的90%[43–44]。而当细胞受到UV辐射后,线粒体中的电子转移不能发生有序的转移,不少电子直接逃逸到氧气中,在线粒体呼吸链的复合体Ⅰ和复合体Ⅲ形成ROS[45–46],造成细胞的损伤及能量代谢的紊乱。
人体可通过自身细胞产生抗氧化剂维持氧稳态,从而抵抗ROS带来的细胞损伤。目前已知的清除体内ROS的抗氧化酶是谷胱甘肽过氧化物酶(glutathione peroxidase,GSH-Px)、超氧化物歧化酶(superoxide dismutase,SOD)和过氧化氢酶(catalase,CAT)[47]。人体通过对氧的动态平衡调控实现氧的能量利用最大化及损伤修复,但在强度过高的UV辐照后,ROS的产生将超越机体系统的调节能力,如高水平的ROS将累积破坏细胞的结构和功能,并介导炎症反应[48–49]。ROS可激活多条细胞信号通路,导致胶原生成减少、基质金属蛋白酶(matrix metalloproteinase,MMPs)合成增多,最终促进皮肤衰老[40]。
1.2.2 脂质过氧化过量UV辐照将产生大量的ROS,而ROS在体内可与不饱和脂肪酸链进行脂质过氧化反应,形成过氧化脂质。过氧化脂质一方面会扰乱磷脂双分子层的结构,影响细胞膜的完整性[50];另一方面,脂质过氧化物可以进一步分解成许多反应醛,与DNA碱基发生反应,产生无法修复的聚合物,最终导致各种皮肤疾病[51–52]。
1.2.3 对蛋白质的损伤长期的UV照射将对真皮细胞外基质(extracellular matrix,ECM)造成明显的损伤。真皮ECM最重要及含量最高的物质是胶原、弹性蛋白和糖胺聚糖,这些蛋白的主要功能是维持皮肤的强度、弹性和水分[53]。光老化导致的皮肤粗糙、松弛、起皱与皮肤中MMPs的增加密切相关。MMPs的是一类锌依赖的内肽酶家族,其主要生理作用是降解ECM中的各种蛋白质成分[54]。在光老化皮肤中,不仅MMPs的表达增加,其拮抗因子——金属蛋白酶组织抑制因子(tissue inhibitor of matrix metalloproteinases,TIMPs)的表达也降低[55–56]。
另外,导致ECM结构损伤的主要因素就是ROS水平的上升。UV照射后皮肤细胞内ROS增加,DNA、脂质和蛋白质的氧化产物增多,这
些产物能激活细胞因子,使细胞内凋亡相关信号转导通路如MAPK、ERK等激活[57],然后激活转录因子AP-1和NF-κB。一方面,AP-1和NF-κB上调MMPs的转录水平、下调TIMPs的转录水平,引起胶原蛋白被降解[58];另一方面,皮肤细胞的AP-1和NF-κB可下调转化生长因子-β (transforming growth factor-β,TGF-β) Ⅱ型受体的表达,导致下游Smad/TGF-β信号通路受损,降低编码Ⅰ型和Ⅱ型胶原前体的COL3A1和COL1A1基因的转录,减少胶原的生物合成[59−60]。同时,也有研究提出ROS可诱导皮肤组织的弹性蛋白酶活性升高、导致胶原纤维蛋白的异常合成,最终导致ECM内的无定形弹性蛋白增多,皮肤弹性下降。因此,在光老化的皮肤中,可观察到异常弹性蛋白的沉积、弹性物质聚集体的弯曲、紊乱和高度分支的弹性纤维[61]。
1.2.4 炎症反应UV辐照除了通过DNA、RNA及蛋白损伤外,也会诱发皮肤炎症,导致皮肤光老化。UV通过引起皮肤细胞内ROS产生,导致细胞释放白细胞介素家族IL-1、IL-6、IL-8、IL-10和TNF-α等系列炎症介质[62],从而使局部皮肤的炎症发生。
2 益生菌的抗光老化作用 2.1 益生菌的抗氧化作用益生菌是一类对人体有益的活性微生物,具有维持肠道菌群结构平衡、调节机体免疫力、提高机体抗氧化水平等功能。其中,益生菌的抗氧化功能与皮肤系统的健康密切相关。益生菌的抗氧化机制可以分为4种:清除活性氧和自由基系统、增强抗氧化酶系统、还原调控系统和氧化损伤修复系统[63]。由于益生菌可以通过多种途径协助机体抗氧化,因此不少研究者试图将益生菌应用于皮肤抗光老化的治疗中(表 1)。
| Strains | Form | Test sample and quantity | Mechanism | Effect |
| Lactobacillus plantarum GDMCC61123 | Live bacteria | C57 mice: 10 controls vs 10 models vs 10 tests |
Increased the activities of GSH-Px and SOD in mice | Strong free radical scavenging ability and anti-lipid peroxidation ability[26] |
| Lactobacillus acidophilus KCCM12625 | Inactivated microorganisms | HaCaT/HDF Cells | Increase the activity of skin antioxidant enzymes such as SOD and CAT; reduce the expression levels of MMP-1 and MMP-9 produced in the process of skin aging, and increase the expression of procollagen | Reduced the loss of collagen in the dermis and prevented skin oxidative stress damage caused by UVB[65] |
| Bifidobacterium breve Yakult | Live bacteria | female hairless mice (Hos: HR-1 strain): 6 controls vs 6 models vs 6 tests |
Reduced the production of ROS; reduced the activity of elastase in the skin | Reduced skin barrier disturbance and oxidative stress caused by UV[66]; resistant to skin damage caused by UV[75] |
| Limosilactobacillus fermentum GDMCC 61827 | Cell-free metabolites | HaCaT Cells/Dunkin Hartley Guinea pig: 6 controls vs 6 models vs 6 tests |
Reduce the production of ROS in cells and stabilize mitochondrial membrane potential; down-regulate the transcription levels of MMP-1, MMP-3, IL-1β, IL-6, and IL-8 in skin cells damaged by UVB | Reduced collagen degradation in vitro and in vivo; relieved mild cell death and inflammatory cell infiltration in the skin of guinea pigs irradiated by UV[67] |
| Lactobacillus helveticus NS-8 | Fermented milk | SKH-1 hairless mice: 10 controls vs 10 models vs 10 tests |
Scavenged free radicals, reduced UV-induced ROS; improved the activity level of skin peroxidase | Alleviated the photoaging effect of skin cells[68] |
| Lactobacillus rhamnosus HK-9 | Fermented plant extract | HaCaT Cells | Significantly inhibited the production of ROS in HaCaT Cells; improved the anti-MMP-2 and MMP-9 formation ability of agastache leaves | Improved the anti-photoaging activity of natural products[69] |
| Lactobacillus plantarum C2 | Fermented plant extract | Mouse fibroblasts Cells Balb3T3 | Increased the content of antioxidants such as total phenols, flavonoids and anthocyanins | Enhanced the scavenging ability of free radicals and inhibit the peroxidation ability of linoleic acid[70] |
| Lactobacillus plantarum HY7714 | Inactivated microorganisms | Hs68 HDF Cells | Inhibited the activation of JNK/AP-1 signaling pathway and reduced the excessively high MMP-13 transcription level and MMP-2 and MMP-9 activities in UVB damaged cells | Reduced collagen degradation[72] |
| Lactobacillus sakei KCCM 11175P | Microbial structure composition | HNDF Cells | Blocked MAPK signal and inhibited the expression of MMP-1 transcription factor AP-1 | Increased the content of collagen in dermal cells and relievied photoaging damage[73] |
| Lactobacillus casei B9-1 | Microbial structure composition | Normal primary human fibroblast | Extracellular polysaccharides down-regulated the expression levels of MMP-1, MMP-2, MMP-3, MMP-9 and MMP-10, and up-regulated TIMPs; enhanced the anti-collagenase and anti-elastase activities in skin cells | Effectively reduce collagen degradation by UV[74] |
| Lactobacillus brucei JCM1115 | Fermented plant extract | CCD-986sk fibroblasts and HaCaT Cells | Effectively inhibit UVB-induced elastase activity and MMPs expression | Promoted the synthesis of type Ⅰ procollagen[76] |
| Lactobacillus acidophilus IDCC 3302 | Inactivated microorganisms | HaCaT Cells | Inhibited the production of pro-inflammatory cytokines mediated by MAPK signaling pathway | Reduced skin inflammation caused by UVB[64] |
| Lactobacillus reuteri DSM17938 | Live bacteria/microbial lysates | Reconstructed human epidermis | Reduced the expression level of pro-inflammatory factors IL-6 and IL-8; increased the level of laminin A/B | Anti-inflammatory and improve skin barrier function[83] |
| Bifidobacterium breve B-3 | Live bacteria | Hairless mice (Hos: HR-1 strain): 8 controls vs 8 models vs 8 tests | Reduced UV-induced interleukin-1β production in the skin | Reduce inflammation in mice irradiated by UV[85] |
部分益生菌菌株可以增加抗氧化酶活性或清除ROS,减轻皮肤在UV辐照后的氧化损伤(图 1)。作者所在研究团队发现植物乳杆菌GDMCC61123具有较强的自由基清除能力和抗脂质过氧化能力,体内实验结果证明该菌株可以增加小鼠体内谷胱甘肽过氧化物酶和超氧化物歧化酶活性,缓解体内氧化应激作用明显[26]。Im等发现嗜酸乳杆菌IDCC 3302通过增加皮肤抗氧化酶如超氧化物歧化酶和过氧化氢酶的活性防止UVB引起的氧化应激损伤[64]。Lim等通过ABTS自由基清除试验发现嗜酸乳杆菌KCCM12625具有较好的抗氧化作用,且能显著降低UVB辐射后HaCaT细胞中升高的ROS水平,减轻氧化损伤导致的光老化现象[65]。Ishii等通过体内实验证明口服短双歧杆菌Yakult可防止ROS的产生,减弱UV引起的皮肤屏障扰动和氧化应激[66]。说明益生菌产品在抗氧化、抗皮肤光老化治疗中有良好的开发前景。
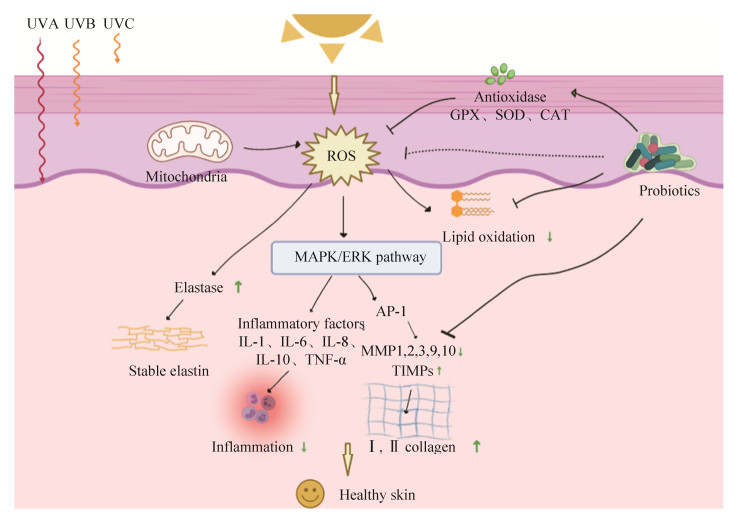
|
| 图 1 益生菌对皮肤光老化的修复通路 Figure 1 The repair pathway of probiotics on skin photoaging. |
由于含活菌的制剂在日化用品中受到一定的限制,所以有不少研究者尝试验证益生菌的发酵产物是否同样具有抗氧化作用。作者所在研究团队发现发酵乳杆菌GDMCC 61827的发酵上清具有良好的抗UV诱发的皮肤光损伤能力,采用比较基因组学及靶向代谢组证实该发酵乳杆菌具有良好的烟酰胺合成功能,发酵液中含较高的烟酰胺。发酵乳杆菌GDMCC 61827的发酵上清作用于UV损伤的皮肤细胞后能明显减少细胞内活性氧的产生、高效稳定线粒体膜电位,在体外及体内均展现良好的抗光老化作用[67]。瑞士乳杆菌NS-8发酵液具有显著的清除自由基的功能,可降低UV诱导生成的ROS,改善皮肤过氧化酶的活性水平,缓解皮肤细胞光损伤作用[68]。
此外,亦有研究者尝试将益生菌用于植物天然成分的提取中,以增加植物提取物的抗氧化特性。Lim等通过比较未发酵和鼠李糖乳杆菌HK-9发酵的龙舌兰叶提取物的皮肤抗氧化特性,发现采用HK-9发酵后的龙舌兰叶提取物能显著抑制角质形成细胞中活性氧如NO和iNOS的产生,证明采用益生菌能提高天然产物的抗光老化活性[69]。Curiel等发现采用植物乳杆菌C2发酵桃金娘浆可明显提高匀浆中的抗氧化物质如总酚、黄酮和花色苷的浓度,增强其对自由基的清除力和抑制亚油酸的过氧化能力[70]。
益生菌可通过菌体自身、发酵上清以及发酵其他天然成分发挥抗氧化作用,在协助机体防御光老化中有良好的应用前景。
2.2 益生菌减少UV对真皮细胞外基质的破坏真皮ECM损伤如胶原蛋白流失和弹性蛋白下降是皮肤光老化过程中一个重要的病理特征。在光老化的皮肤中,UV辐照后会使皮肤细胞内的ROS增多,诱发MMPs水平增高,导致皮肤的胶原蛋白和弹性蛋白被降解,皮肤变得粗糙、松弛和起皱。益生菌除了能显著降低紫外辐射诱导的ROS水平之外,还能通过影响多个信号通路直接调节皮肤细胞MMPs的表达水平,减少UV辐照后胶原蛋白和弹性蛋白的降解[71]。作者所在研究团队发现发酵乳杆菌GDMCC 61827可下调UVB损伤导致的皮肤细胞中MMP-1、MMP-3转录水平上升,从而缓解皮肤光老化症状。Lim等发现嗜酸乳杆菌KCCM 12625可以通过影响皮肤细胞AP-1信号通路,减少皮肤衰老过程中产生的MMP-1、MMP-9的mRNA的表达,同时增加前胶原蛋白的表达,减少真皮层胶原蛋白流失[65]。Kim等也证明了植物乳酸菌HY7714通过抑制JNK/AP-1信号通路的激活而降低了UVB损伤细胞内过高的MMP-13转录水平和MMP-2、MMP-9活性[72]。有学者推测,益生菌的结构多糖是其发挥抗光老化作用的主要功效成分。脂磷壁酸(lipoteichoic acid,LTA)是革兰氏阳性菌细胞壁的一种成分,You发现清酒乳杆菌LTA可通过阻断MAPK信号抑制MMP-1转录因子AP-1的表达,增加真皮细胞胶原蛋白的含量,延缓光老化损伤[73]。Shirzad等应用干酪乳杆菌B9-1的胞外多糖作用于UV损伤的皮肤细胞,同样发现其具有下调MMP-1、MMP-2、MMP-3、MMP-9和MMP-10表达水平、上调TIMPs的作用,发现干酪乳杆菌B9-1可增强皮肤细胞中抗胶原酶、抗弹性蛋白酶活性,有效减少光照后胶原蛋白的降解[74]。益生菌的抗光老化效果除了在细胞水平上得到验证之外,在动物水平上也同样得到验证。Kano等发现无毛小鼠口服短双歧杆菌Yakult后能显著抵抗UV辐射引起的皮肤损伤,表现为皮肤中弹性蛋白酶的活性下降,皮肤弹性较不服用益生菌的小鼠明显增加[75]。
益生菌发酵其他植物可提高天然产物的抗光老化活性。Kang等在泡菜中发现采用布氏乳杆菌发酵植物获得的提取物,可有效抑制UVB诱导的弹性蛋白酶活性和MMPs的表达,促进Ⅰ型前胶原的合成[76]。Shin等采用乳酸菌发酵藿香叶,证明能明显提高藿香叶抗MMP-2和MMP-9形成的能力[77]。
综上所述,益生菌可通过多种途径减少光老化过程中胶原蛋白和弹性蛋白的流失,继而达到延缓光老化的效果。
2.3 益生菌抑制炎症因子的表达除了上述造成光老化组织学差异的因素之外,炎症因子的累积也是光老化过程中皮肤损伤的一个不可忽视的因素,皮肤炎症因子的增多会导致屏障功能受到破坏,表皮水分失衡、皮肤通透性增加,引起更多的过敏原及化学物质的异常吸入,引起或加重皮肤疾病状态[78],加速皮肤老化。而益生菌在缓解肠道炎症上已有广泛的应用,如作者所在研究团队发现戊糖片球菌IM96能显著缓解并降低机体在感染大肠杆菌O157:H7后的炎症水平[79]。同样地,益生菌缓解炎症的功能在调控皮肤免疫平衡上也有良好的疗效,如益生菌在特应性皮炎的治疗上已被广泛应用,证明益生菌可有效降低患者皮肤炎症水平,恢复免疫稳态[80]。
在UV辐射下,表皮层的角质形成细胞通过释放白细胞介素IL-1、IL-6、IL-8、IL-10和TNF-α等炎症介质,诱发局部的炎症,导致皮肤通透性增加,屏障功能下降[81-82]。嗜酸乳杆菌IDCC 3302可以抑制MAPK信号通路介导的促炎细胞因子的产生,减少UVB辐射引起的皮肤炎症[64]。Khmaladze等发现罗伊氏乳杆菌可缓解UV辐照诱导的皮肤炎症[83]。Satoh等证实口服益生菌同样能减少炎症、抵抗光老化:受UV辐照的小鼠在服用短双歧杆菌B-3后,能有效减少皮肤中紫外线诱导的白细胞介素-1β的产生[84]。作者所在研究团队发现发酵乳杆菌GDMCC 61827可下调UVB损伤导致皮肤细胞中IL-1β、IL-6、IL-8表达水平的上升,并且外敷发酵乳杆菌GDMCC 61827发酵上清液能有效缓解紫外辐照豚鼠皮肤中轻度细胞死亡和炎症细胞浸润情况。Keshari等发现新一代益生菌表皮葡萄球菌能通过发酵代谢产物中的丁酸介导短链脂肪酸受体2表达,调节UVB诱导的促炎细胞因子IL-6的产生[85]。
因此,益生菌可通过抑制UV辐照后皮肤异常免疫作用的形成,减少皮肤的光老化损伤。
3 皮肤应用益生菌的潜在形式随着人们对皮肤微生物区系了解的不断加深,我们相信会有更多针对个体特征定制的皮肤护理方案出现。但是在益生菌的皮肤应用中还存在很多待解决的问题:口服和外涂哪种才是最好的应用方式?活性微生物应用效果会比灭活微生物要好吗?如果是,应如何安全和持久地植入益生菌?活菌存活时间有多久?多种益生菌混合物应用是否具有协同作用?
人们通常认为活性益生菌比灭活益生菌更有可能具有生物活性,因为活性益生菌比灭活细胞更容易附着在宿主黏膜和细胞上,并且可对有害微生物产生更好的抗菌活性[86]。因此,最理想的皮肤益生菌治疗方案应该为让有活性的益生菌在皮肤上保持活性,并在皮肤上定居[87]。但很少有研究表明局部使用的益生菌可以在皮肤上增殖、定居,并融入宿主正常皮肤微生物区系的成员。如果采用口服摄入益生菌,则难以保证益生菌的长期定植,停用益生菌后功效将很快消失[88]。因此,如何维持益生菌活菌的持久性将是研究者们关注的新方向。
至今,大多数皮肤益生菌产品并不真的含有活菌,而是由灭活微生物、菌体成分或益生菌的无细胞代谢物组成。目前国内化妆品行业国标要求对产品的菌落总数进行控制,是因为在皮肤上直接应用活菌菌体存在一定风险,例如在皮肤存在伤口的情况下,应用活菌可能会导致细菌移行到血液中,产生菌血症[89]。另外,由于益生菌的保藏和运输方式比较严格,保持活菌在皮肤上存活及生长仍是难以解决的技术问题[31]。目前的科学研究仅从灭活微生物、菌体成分或益生菌的无细胞代谢物对皮肤疾病(如光老化)的分子作用机制和表型进行探讨,而未进一步探究其与皮肤微生态之间的关系。灭活的益生菌、菌体成分或益生菌的无细胞代谢物依然是目前在皮肤上应用益生菌产品首选的应用模式[90]。
4 结论综上所述,UV辐射对皮肤中的DNA、RNA及蛋白质产生直接损伤,影响细胞存活状态;UV同时诱导细胞内ROS累积,导致脂质过氧化发生,进一步损伤DNA和RNA、导致细胞外基质胶原蛋白和弹性蛋白降解;另外UV还诱发皮肤局部炎症,降低皮肤对外界有害物质的防御;这些共同促进了皮肤光老化的发生。而通过应用具有抗氧化功能的益生菌,可以清除活性氧、调节基质金属蛋白酶表达水平,实现缓解细胞外基质损伤,减轻皮肤炎症。益生菌在保护皮肤免受UV损伤方面具有良好的应用前景,随着科学研究的不断深入,研究者们将进一步验证益生菌在维护皮肤健康上的应用价值。因此,在目前的研究基础上,我们还需要从皮肤微生态、益生菌、益生元及后生元的抗光老化分子机制等多方面深入探索益生菌对皮肤系统的作用机制,开发更多新型微生态策略来保护受UV辐射的皮肤,延缓光老化。
| [1] | Jabłońska-Trypuć A, Krętowski R, Kalinowska M, Świderski G, Cechowska-Pasko M, Lewandowski W. Possible mechanisms of the prevention of doxorubicin toxicity by cichoric acid-antioxidant nutrient. Nutrients, 2018, 10(1): 44. DOI:10.3390/nu10010044 |
| [2] | Blume-Peytavi U, Kottner J, Sterry W, Hodin MW, Griffiths TW, Watson REB, Hay RJ, Griffiths CEM. Age-associated skin conditions and diseases: current perspectives and future options. The Gerontologist, 2016, 56(Suppl 2): S230-S242. DOI:10.1093/geront/gnw003 |
| [3] | Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell, 2008, 7(2): 250-259. DOI:10.1111/j.1474-9726.2008.00372.x |
| [4] | Morita A. Tobacco smoke causes premature skin aging. Journal of Dermatological Science, 2007, 48(3): 169-175. DOI:10.1016/j.jdermsci.2007.06.015 |
| [5] | Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S. Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells International, 2016, 2016: 7370642. |
| [6] | Cavinato M, Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Experimental Gerontology, 2017, 94: 78-82. DOI:10.1016/j.exger.2017.01.009 |
| [7] | Han A, Chien AL, Kang S. Photoaging. Dermatologic Clinics, 2014, 32(3): 291-299. DOI:10.1016/j.det.2014.03.015 |
| [8] | Friedman O. Changes associated with the aging face. Facial Plastic Surgery Clinics of North America, 2005, 13(3): 371-380. DOI:10.1016/j.fsc.2005.04.004 |
| [9] | Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the aging skin. Advances in Wound Care, 2013, 2(1): 5-10. DOI:10.1089/wound.2011.0356 |
| [10] | Varani J, Schuger L, Dame MK, Leonard C, Fligiel SEG, Kang S, Fisher GJ, Voorhees JJ. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. Journal of Investigative Dermatology, 2004, 122(6): 1471-1479. DOI:10.1111/j.0022-202X.2004.22614.x |
| [11] | Watson REB, Gibbs NK, Griffiths CEM, Sherratt MJ. Damage to skin extracellular matrix induced by UV exposure. Antioxidants & Redox Signaling, 2014, 21(7): 1063-1077. |
| [12] | Hashizume H. Skin aging and dry skin. The Journal of Dermatology, 2004, 31(8): 603-609. DOI:10.1111/j.1346-8138.2004.tb00565.x |
| [13] | Goukassian DA, Gilchrest BA. The interdependence of skin aging, skin cancer, and DNA repair capacity: a novel perspective with therapeutic implications. Rejuvenation Research, 2004, 7(3): 175-185. DOI:10.1089/rej.2004.7.175 |
| [14] | Ladizinski B, McLean R, Lee KC, Elpern DJ, Eron L. The human skin microbiome. International Journal of Dermatology, 2014, 53(9): 1177-1179. DOI:10.1111/ijd.12609 |
| [15] | Scharschmidt TC, Fischbach MA. What lives on our skin: ecology, genomics and therapeutic opportunities of the skin microbiome. Drug Discovery Today: Disease Mechanisms, 2013, 10(3/4): e83-e89. |
| [16] | Li ZC, Bai XZ, Peng TW, Yi XW, Luo L, Yang JZ, Liu JQ, Wang YC, He T, Wang XJ, Zhu HY, Wang HT, Tao K, Zheng Z, Su LL, Hu DH. New insights into the skin microbial communities and skin aging. Frontiers in Microbiology, 2020, 11: 565549. DOI:10.3389/fmicb.2020.565549 |
| [17] | Fuentes-Tristan S, Parra-Saldivar R, Iqbal HMN, Carrillo-Nieves D. Bioinspired biomolecules: mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities. Journal of Photochemistry and Photobiology B: Biology, 2019, 201: 111684. DOI:10.1016/j.jphotobiol.2019.111684 |
| [18] | Martínez-Navarro FJ, Martínez-Morcillo FJ, López-Muñoz A, Pardo-Sánchez I, Martínez-Menchón T, Corbalán-Vélez R, Cayuela ML, Pérez-Oliva AB, García-Moreno D, Mulero V. The vitamin B6-regulated enzymes PYGL and G6PD fuel NADPH oxidases to promote skin inflammation. Developmental & Comparative Immunology, 2020, 108: 103666. |
| [19] | Cruickshank-Quinn C, Armstrong M, Powell R, Gomez J, Elie M, Reisdorph N. Determining the presence of asthma-related molecules and salivary contamination in exhaled breath condensate. Respiratory Research, 2017, 18(1): 57. DOI:10.1186/s12931-017-0538-5 |
| [20] | De Luca C, Valacchi G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediators of Inflammation, 2010, 2010: 321494. |
| [21] | Guéniche A, Philippe D, Bastien P, Blum S, Buyukpamukcu E, Castiel-Higounenc I. Probiotics for photoprotection. Dermato-endocrinology, 2009, 1(5): 275-279. DOI:10.4161/derm.1.5.9849 |
| [22] | Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clinical Microbiology Reviews, 2003, 16(4): 658-672. DOI:10.1128/CMR.16.4.658-672.2003 |
| [23] | Benno P, Alam M, Bjorklund NE, Kleinau S, Olhagen B, Midtvedt T. Host factors in susceptibility to arthritis induced with adjuvants (M-tuberculosis) or type Ⅱ collagen in Agus rats. Scandinavian Journal of Laboratory Animal Science, 1996, 23: 411-414. |
| [24] | Liang TT, Xie XQ, Zhang JM, Ding Y, Wu QP. Bacterial community and composition of different traditional fermented dairy products in China, South Africa, and Sri Lanka by high-throughput sequencing of 16S rRNA genes. LWT, 2021, 144: 111209. DOI:10.1016/j.lwt.2021.111209 |
| [25] | Liang TT, Xie XQ, Ma J, Wu L, Xi Y, Zhao H, Li LY, Li HX, Feng Y, Xue L, Chen MT, Chen XF, Zhang JM, Ding Y, Wu QP. Microbial communities and physicochemical characteristics of traditional dajiang and sufu in North China revealed by high-throughput sequencing of 16S rRNA. Frontiers in Microbiology, 2021, 12: 665243. DOI:10.3389/fmicb.2021.665243 |
| [26] | 吴清平, 吴磊, 李滢, 梁婷婷, 谢新强, 张菊梅, 丁郁, 王涓, 陈谋通, 薛亮, 叶清华. 一种乳酸菌及其应用: CN112300962A[P]. 2021-02-02. |
| [27] | Kalliomäki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. The Lancet, 2001, 357(9262): 1076-1079. DOI:10.1016/S0140-6736(00)04259-8 |
| [28] | Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infection and Immunity, 1996, 64(12): 5403-5405. DOI:10.1128/iai.64.12.5403-5405.1996 |
| [29] | Borruel N, Casellas F, Antolı́n M, Llopis M, Carol M, Espı́in E, Naval J, Guarner F, Malagelada JR. Effects of nonpathogenic bacteria on cytokine secretion by human intestinal mucosa. The American Journal of Gastroenterology, 2003, 98(4): 865-870. DOI:10.1111/j.1572-0241.2003.07384.x |
| [30] | QB/T 2872-2017中华人民共和国轻工行业标准. 中华人民共和国工业和信息化部. 2017-04-12 |
| [31] | McLoughlin IJ, Wright EM, Tagg JR, Jain R, Hale JDF. Skin microbiome-the next frontier for probiotic intervention. Probiotics and Antimicrobial Proteins, 2021. DOI:10.1007/s12602-021-09824-1 |
| [32] | Parisi AV, Turner J. Variations in the short wavelength cut-off of the solar UV spectra. Photochemical & Photobiological Sciences, 2006, 5(3): 331-335. |
| [33] | Makrantonaki E, Eckardt R, Steinhagen-Thiessen E, Gschnell M, Zouboulis CC. Vom leben gezeichnet. MMW - Fortschritte Der Medizin, 2013, 155(25): 50-57. DOI:10.1007/s15006-013-2130-3 |
| [34] | Maddodi N, Jayanthy A, Setaluri V. Shining light on skin pigmentation: the darker and the brighter side of effects of UV radiation. Photochemistry and Photobiology, 2012, 88(5): 1075-1082. DOI:10.1111/j.1751-1097.2012.01138.x |
| [35] | Gibbs NK, Norval M. Photoimmunosuppression: a brief overview. Photodermatology, Photoimmunology & Photomedicine, 2013, 29(2): 57-64. |
| [36] | Chen HX, Weng QY, Fisher DE. UV signaling pathways within the skin. Journal of Investigative Dermatology, 2014, 134(8): 2080-2085. DOI:10.1038/jid.2014.161 |
| [37] | Goodsell DS. The molecular perspective: ultraviolet light and pyrimidine dimers. The Oncologist, 2001, 6(3): 298-299. DOI:10.1634/theoncologist.6-3-298 |
| [38] | Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Pontén J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proceedings of the National Academy of Sciences of the United States of America, 1991, 88(22): 10124-10128. DOI:10.1073/pnas.88.22.10124 |
| [39] | Ljungman M, Zhang F. Blockage of RNA polymerase as a possible trigger for u.v. light-induced apoptosis. Oncogene, 1996, 13(4): 823-831. |
| [40] | Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Research Reviews, 2015, 21: 16-29. DOI:10.1016/j.arr.2015.01.001 |
| [41] | Parrish JA, Fitzpatrick TB, Tanenbaum L, Pathak MA. Photochemotherapy of psoriasis with oral methoxsalen and longwave ultraviolet light. The New England Journal of Medicine, 1974, 291(23): 1207-1211. DOI:10.1056/NEJM197412052912301 |
| [42] | Ghosh M, Das J, Sil PC. D(+) galactosamine induced oxidative and nitrosative stress-mediated renal damage in rats via NF-κB and inducible nitric oxide synthase (iNOS) pathways is ameliorated by a polyphenol xanthone, mangiferin. Free Radical Research, 2012, 46(2): 116-132. DOI:10.3109/10715762.2011.644240 |
| [43] | Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nature Reviews Molecular Cell Biology, 2014, 15(6): 411-421. DOI:10.1038/nrm3801 |
| [44] | Naidoo K, Hanna R, Birch-Machin MA. What is the role of mitochondrial dysfunction in skin photoaging?. Experimental Dermatology, 2018, 27(2): 124-128. DOI:10.1111/exd.13476 |
| [45] | Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annual Review of Pharmacology and Toxicology, 2007, 47: 143-183. DOI:10.1146/annurev.pharmtox.47.120505.105122 |
| [46] | Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan JY. Caspase-12 mediates endoplasmic-Reticulum-specific apoptosis and cytotoxicity by amyloid-Β. Nature, 2000, 403(6765): 98-103. DOI:10.1038/47513 |
| [47] | Pinnell SR. Cutaneous photodamage, oxidative stress, and topical antioxidant protection. Journal of the American Academy of Dermatology, 2003, 48(1): 1-22. DOI:10.1067/mjd.2003.16 |
| [48] | Petruk G, Del Giudice R, Rigano MM, Monti DM. Antioxidants from plants protect against skin photoaging. Oxidative Medicine and Cellular Longevity, 2018, 2018: 1454936. |
| [49] | Sajo MEJ, Kim CS, Kim SK, Shim KY, Kang TY, Lee KJ. Antioxidant and anti-inflammatory effects of shungite against ultraviolet B irradiation-induced skin damage in hairless mice. Oxidative Medicine and Cellular Longevity, 2017, 2017: 7340143. |
| [50] | Girotti AW. Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. Journal of Photochemistry and Photobiology B: Biology, 2001, 63(1/2/3): 103-113. |
| [51] | Marnett LJ. Lipid peroxidation—DNA damage by malondialdehyde. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 1999, 424(1/2): 83-95. |
| [52] | Niki E. Lipid oxidation in the skin. Free Radical Research, 2015, 49(7): 827-834. DOI:10.3109/10715762.2014.976213 |
| [53] | Oxlund H, Andreassen TT. The roles of hyaluronic acid, collagen and elastin in the mechanical properties of connective tissues. Journal of Anatomy, 1980, 131(4): 611-620. |
| [54] | Birkedal-Hansen H. [8] catabolism and turnover of collagens: collagenases. Methods in Enzymology, 1987, 144: 140-171. |
| [55] | Afaq F, Katiyar SK. Polyphenols: skin photoprotection and inhibition of photocarcinogenesis. Mini Reviews in Medicinal Chemistry, 2011, 11(14): 1200-1215. |
| [56] | Lephart ED, Sommerfeldt JM, Andrus MB. Resveratrol: influences on gene expression in human skin. Journal of Functional Foods, 2014, 10: 377-384. DOI:10.1016/j.jff.2014.07.017 |
| [57] | Xu YR, Shao Y, Voorhees JJ, Fisher GJ. Oxidative inhibition of receptor-type protein-tyrosine phosphatase κ by ultraviolet irradiation activates epidermal growth factor receptor in human keratinocytes. Journal of Biological Chemistry, 2006, 281(37): 27389-27397. DOI:10.1074/jbc.M602355200 |
| [58] | Hall MC, Young DA, Waters JG, Rowan AD, Chantry A, Edwards DR, Clark IM. The comparative role of activator protein 1 and smad factors in the regulation of Timp-1 and MMP-1 gene expression by transforming growth factor-β1. Journal of Biological Chemistry, 2003, 278(12): 10304-10313. DOI:10.1074/jbc.M212334200 |
| [59] | Kim J, Takahashi M, Shimizu T, Shirasawa T, Kajita M, Kanayama A, Miyamoto Y. Effects of a potent antioxidant, platinum nanoparticle, on the lifespan of Caenorhabditis elegans. Mechanisms of Ageing and Development, 2008, 129(6): 322-331. DOI:10.1016/j.mad.2008.02.011 |
| [60] | Quan TH, He TY, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-β type Ⅱ receptor/smad signaling. The American Journal of Pathology, 2004, 165(3): 741-751. DOI:10.1016/S0002-9440(10)63337-8 |
| [61] | Naylor EC, Watson REB, Sherratt MJ. Molecular aspects of skin ageing. Maturitas, 2011, 69(3): 249-256. DOI:10.1016/j.maturitas.2011.04.011 |
| [62] | Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. Journal of Photochemistry and Photobiology B: Biology, 2001, 63(1/2/3): 8-18. |
| [63] | 赵吉春. 植物乳杆菌抗氧化评价及抗氧化机制研究. 江南大学博士学位论文, 2018. |
| [64] | Im AR, Lee B, Kang DJ, Chae S. Protective effects of tyndallized Lactobacillus acidophilus IDCC 3302 against UVB-induced photodamage to epidermal keratinocytes cells. International Journal of Molecular Medicine, 2019, 43(6): 2499-2506. |
| [65] | Lim HY, Jeong D, Park SH, Shin KK, Hong YH, Kim E, Yu YG, Kim TR, Kim H, Lee J, Cho JY. Antiwrinkle and antimelanogenesis effects of tyndallized Lactobacillus acidophilus KCCM12625P. International Journal of Molecular Sciences, 2020, 21(5): 1620. DOI:10.3390/ijms21051620 |
| [66] | Ishii Y, Sugimoto S, Izawa N, Sone T, Chiba K, Miyazaki K. Oral administration of Bifidobacterium breve attenuates UV-induced barrier perturbation and oxidative stress in hairless mice skin. Archives of Dermatological Research, 2014, 306(5): 467-473. DOI:10.1007/s00403-014-1441-2 |
| [67] | 吴清平, 陈慧贞, 李滢, 谢新强, 张菊梅, 杨宁, 陈惠元, 代京莎, 陈玲, 刘振杰. 一株高效合成烟酰胺、抗光老化的发酵乳杆菌及其应用: CN113832050A[P]. 2021-12-24. |
| [68] | 单闯. 瑞士乳杆菌发酵液NS-FS干预皮肤光衰老的作用及机制研究. 杭州师范大学硕士学位论文, 2016. |
| [69] | Lim HW, Lee Y, Huang YH, Yoon JY, Lee SH, Kim K, Lim CJ. Enhancement of skin antioxidant and anti-inflammatory potentials of Agastache rugosa leaf extract by probiotic bacterial fermentation in human epidermal keratinocytes. Microbiology and Biotechnology Letters, 2017, 45(1): 35-42. DOI:10.4014/mbl.1701.01002 |
| [70] | Curiel JA, Pinto D, Marzani B, Filannino P, Farris GA, Gobbetti M, Rizzello CG. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microbial Cell Factories, 2015, 14: 67. DOI:10.1186/s12934-015-0250-4 |
| [71] | Lavker RM, Zheng PS, Dong G. Morphology of aged skin. Clinics in Geriatric Medicine, 1989, 5(1): 53-67. DOI:10.1016/S0749-0690(18)30695-5 |
| [72] | Kim HM, Lee DE, Park SD, Kim YT, Kim YJ, Jeong JW, Jang SS, Ahn YT, Sim JH, Huh CS, Chung DK, Lee JH. Oral administration of Lactobacillus plantarum HY7714 protects hairless mouse against ultraviolet B-induced photoaging. Journal of Microbiology and Biotechnology, 2014, 24(11): 1583-1591. DOI:10.4014/jmb.1406.06038 |
| [73] | You GE. Lactobacillus sakei lipoteichoic acid inhibits MMP-1 induced by UVA in normal dermal fibroblasts of human. Journal of Microbiology and Biotechnology, 2013, 23(10): 1357-1364. DOI:10.4014/jmb.1306.06026 |
| [74] | Shirzad M, Hamedi J, Motevaseli E, Modarressi MH. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artificial Cells, Nanomedicine, and Biotechnology, 2018, 46(sup1): 1051-1061. DOI:10.1080/21691401.2018.1443274 |
| [75] | Kano M, Masuoka N, Kaga C, Sugimoto S, Iizuka R, Manabe K, Sone T, Oeda K, Nonaka C, Miyazaki K, Ishikawa F. Consecutive intake of fermented milk containing Bifidobacterium breve strain yakult and galacto-oligosaccharides benefits skin condition in healthy adult women. Bioscience of Microbiota, Food and Health, 2013, 32(1): 33-39. DOI:10.12938/bmfh.32.33 |
| [76] | Kang YM, Hong CH, Kang SH, Seo DS, Kim SO, Lee HY, Sim HJ, An HJ. Anti-photoaging effect of plant extract fermented with Lactobacillus buchneri on CCD-986sk fibroblasts and HaCaT keratinocytes. Journal of Functional Biomaterials, 2020, 11(1): 3. DOI:10.3390/jfb11010003 |
| [77] | Shin D, Lee Y, Huang YH, Lim HW, Jang K, Kim DD, Lim CJ. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complementary and Alternative Medicine, 2018, 18(1): 196. DOI:10.1186/s12906-018-2194-9 |
| [78] | Segre JA. Epidermal barrier formation and recovery in skin disorders. The Journal of Clinical Investigation, 2006, 116(5): 1150-1158. DOI:10.1172/JCI28521 |
| [79] | 吴清平, 李海新, 李滢, 谢新强, 陈惠元, 张菊梅, 丁郁, 王涓, 陈谋通, 薛亮, 张淑红, 杨小鹃, 韦献虎, 张友雄. 一种拮抗大肠杆菌O157: H7的戊糖片球菌及其应用: CN113528381A[P]. 2021-10-22. |
| [80] | Pelucchi C, Chatenoud L, Turati F, Galeone C, Moja L, Bach JF, la Vecchia C. Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology: Cambridge, Mass, 2012, 23(3): 402-414. DOI:10.1097/EDE.0b013e31824d5da2 |
| [81] | Kupper TS, Groves RW. The interleukin-1 axis and cutaneous inflammation. Journal of Investigative Dermatology, 1995, 105(1): S62-S66. DOI:10.1038/jid.1995.13 |
| [82] | Ansel JC, Luger TA, Green I. The effect of in vitro and in vivo UV irradiation on the production of ETAF activity by human and murine keratinocytes. Journal of Investigative Dermatology, 1983, 81(6): 519-523. DOI:10.1111/1523-1747.ep12522862 |
| [83] | Khmaladze I, Butler É, Fabre S, Gillbro JM. Lactobacillus reuteri DSM 17938—A comparative study on the effect of probiotics and lysates on human skin. Experimental Dermatology, 2019, 28(7): 822-828. DOI:10.1111/exd.13950 |
| [84] | Satoh T, Murata M, Iwabuchi N, Odamaki T, Wakabayashi H, Yamauchi K, Abe F, Xiao JZ. Effect of Bifidobacterium breve B-3 on skin photoaging induced by chronic UV irradiation in mice. Beneficial Microbes, 2015, 6(4): 497-504. DOI:10.3920/BM2014.0134 |
| [85] | Keshari S, Balasubramaniam A, Myagmardoloonjin B, Herr DR, Negari IP, Huang CM. Butyric acid from probiotic Staphylococcus epidermidis in the skin microbiome down-regulates the ultraviolet-induced pro-inflammatory IL-6 cytokine via short-chain fatty acid receptor. International Journal of Molecular Sciences, 2019, 20(18): 4477. DOI:10.3390/ijms20184477 |
| [86] | Marteau P, Seksik P. Tolerance of probiotics and prebiotics. Journal of Clinical Gastroenterology, 2004, 38(6 Suppl): S67-S69. |
| [87] | Pfefferle PI, Prescott SL, Kopp M. Microbial influence on tolerance and opportunities for intervention with prebiotics/probiotics and bacterial lysates. Journal of Allergy and Clinical Immunology, 2013, 131(6): 1453-1463. DOI:10.1016/j.jaci.2013.03.020 |
| [88] | Ouwehand AC, Salminen SJ. The health effects of cultured milk products with viable and non-viable bacteria. International Dairy Journal, 1998, 8(9): 749-758. DOI:10.1016/S0958-6946(98)00114-9 |
| [89] | Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, Valtonen V. Safety of probiotics that contain lactobacilli or bifidobacteria. Clinical Infectious Diseases: an Official Publication of the Infectious Diseases Society of America, 2003, 36(6): 775-780. DOI:10.1086/368080 |
| [90] | Patra V, Sérézal IG, Wolf P. Potential of skin microbiome, pro- and/or pre-biotics to affect local cutaneous responses to UV exposure. Nutrients, 2020, 12(6): 1795. DOI:10.3390/nu12061795 |
 2022, Vol. 62
2022, Vol. 62




