中国科学院微生物研究所,中国微生物学会
文章信息
- 黄海宁, 黄乾生. 2022
- HUANG Haining, HUANG Qiansheng.
- 细菌胞外囊泡的研究进展
- Research progress on extracellular vesicles of bacteria
- 微生物学报, 62(5): 1613-1628
- Acta Microbiologica Sinica, 62(5): 1613-1628
-
文章历史
- 收稿日期:2021-09-29
- 修回日期:2021-11-30
- 网络出版日期:2021-12-14
2. 中国科学院城市环境研究所, 城市环境与健康重点实验室, 福建 厦门 361021;
3. 中国科学院大学, 北京 100049;
4. 国家基础学科公共科学数据中心, 北京 100190
2. Key Laboratory of Urban Environment and Health, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, Fujian, China;
3. University of Chinese Academy of Sciences, Beijing 100049, China;
4. National Basic Science Data Center, Beijing 100190, China
胞外囊泡(extracellular vesicles,EVs),又叫膜囊泡(membrane vesicles,MVs),是一种由生物体分泌的,磷脂双分子层膜包被的,包裹核酸、蛋白、脂类等分子的球形纳米颗粒物,其大小通常为30–200 nm [1]。来自古菌、细菌和真核生物这3个生命领域的细胞都能够分泌EVs[2]。不同来源的EVs在产生方式、内含物质等方面具有比较大的差异。
真核细胞产生的EVs有多个亚群,包括外泌体(exosomes,直径为30–150 nm)、微囊泡(microvesicles,直径为100–1 000 nm)和凋亡小体(apoptotic vesicles,直径为50 nm–2 μm)[3–4]。目前针对真核EVs的研究很多,主要集中在疾病诊断和治疗方面的应用潜能[5]、作为细胞间物质和信号通讯的工具[6]及在衰老中的作用[7]。近年来,细菌EVs的研究也开始受到关注,本文就细菌胞外囊泡及其在环境领域的研究进展进行总结。
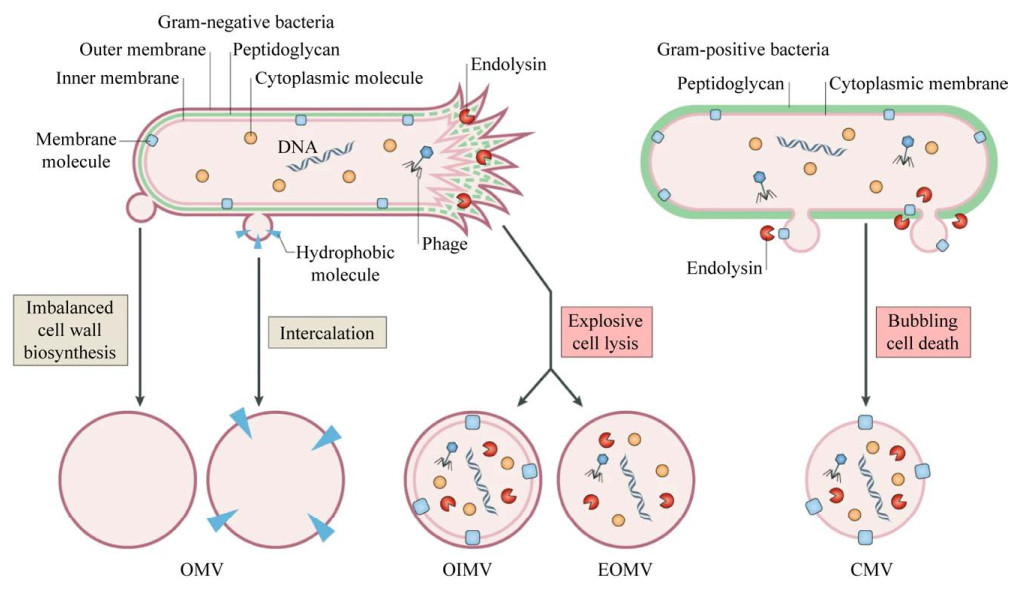
细菌EVs的发现最早可追溯到20世纪60年代[8],近几十年来,对于细菌研究主要集中在革兰氏阴性菌,如大肠埃希菌、鲍曼不动杆菌、铜绿假单胞菌和幽门螺杆菌等。由于革兰氏阳性菌细胞壁较厚,一直被认为无法分泌EVs。直到2007年,研究人员从分枝杆菌中分离出EVs[9],首次证明革兰氏阳性菌也能分泌EVs。近年来,关于革兰氏阳性菌的研究越来越多。细菌分泌的EVs类型多种多样,根据产生方式不同,EVs包括:古菌和革兰氏阳性细菌来源的细胞质膜囊泡(cytoplasmic membrane vesicles,CMVs)、革兰氏阴性细菌来源的外囊泡(outer membrane vesicles,OMVs)、外-内膜囊泡(outer-inner membrane vesicles,OIMVs)和爆炸性外膜囊泡(explosive outer membrane vesicles,EOMVs)[10]。本文将这些不同种类的囊泡统称为“胞外囊泡(extracellular vesicles,EVs)”。
1 细菌EVs形成方式EVs的产生是一个复杂的过程,图 1为不同类型的EVs形成方式的假说。革兰氏阴性菌产生EVs主要通过2种途径:(1) 外膜起泡形成外膜囊泡(OMVs);(2) 由细胞裂解形成外-内膜囊泡(OIMVs)和爆炸性外膜囊泡(EOMVs)[10]。当生物合成肽聚糖不平衡时或疏水性分子插入外膜引起细胞膜紊乱时,导致外膜起泡,产生外膜囊泡(OMVs)。OMVs起源于外膜,因此富含外膜蛋白,并显示特定的脂质组成[11]。爆炸性细胞裂解由两种方式引起:(1) 细胞壁肽聚糖层被自溶素降解,内膜向外突出,DNA等细胞质内容物进入囊泡,最终囊泡与周围的外膜一起从细胞表面被挤压出来,形成外-内膜囊泡(OIMVs)。Pérez-Cruz等使用冷冻透射电镜发现希瓦氏菌(Shewanella vesiculosa M7T)不仅分泌OMVs,同时也分泌结构更复杂的OIMVs,OIMVs在形成过程中沿着内膜和细胞质内容物移动,能够捕获细胞质物质,如DNA[12]。(2) 当细菌染色体DNA受损伤时,会诱导氧化应激反应并触发细胞死亡裂解,导致细胞膜碎片重新循环、聚集并随机包裹细胞质物质,形成爆炸性外膜囊泡(EOMVs)。在内溶酶Lys作用下,铜绿假单胞菌(Pseudomonas aeruginosa)发生爆炸性细胞裂解,Turnbull等通过超分辨率显微镜捕获到细胞膜破碎,这些膜碎片而后迅速形成EOMVs,这些EOMVs随机含有细胞质成分[13]。
革兰氏阳性细菌形成囊泡(CMVs)的途径主要是内溶素触发菌体死亡裂解并释放囊泡,CMVs内部包含细胞膜和细胞质成分[14–15]。Toyofuku等使用活细胞成像和电子低温断层扫描技术研究枯草芽孢杆菌(Bacillus subtilis)中形成胞外囊泡的机制,研究发现,原噬菌体编码的内溶酶表达会在细胞壁肽聚糖层产生孔洞。细胞质中的物质通过这些孔洞向外凸出,并以CMVs的形式释放出来[14]。
2 细菌EVs的分离与鉴定方法 2.1 EVs提取方法真核细胞来源的细胞外囊泡的分离和表征指南已发表在胞外囊泡专业性期刊Journal of Extracellular Vesicles上[3],指南包括从样本收集到分离表征的全部过程。相比之下,目前缺乏原核生物分离EVs的标准。针对原核生物EVs,常用的提取方法包括超速离心法、密度梯度离心法、尺寸排阻色谱法、沉淀法以及亲和层析法。不同的方法有其优势及局限性,表 1总结了目前已用于原核生物EVs提取方法的优缺点。在实验方法选择上,研究者应根据下游实验目的选择合适的方法。
| Methods | Advantages | Disadvantages | References |
| Ultracentrifugation | Simple operation; suitable for large sample volumes | Instrumentation dependent; long lasting | [16] |
| Density gradient ultracentrifugation | High purity | Complex operation; low recovery rate | [17–18] |
| Size exclusion chromatography | Simple operation | Long lasting; not suitable for large sample volumes | [17, 19] |
| Precipitation | Simple and economical; suitable for large sample volumes | Low separation efficiency; low purity | [20–21] |
| Affinity chromatography | High purity | Expensive; not suitable for large sample volumes | [22] |
2.2 EVs鉴定方法
绝大多数文献同时使用2种方法进行EVs的表征:EVs形态表征和EVs颗粒表征(尺寸分布、颗粒浓度)[20]。
2.2.1 EVs形态表征由于EVs尺寸较小,一般的光学显微镜分辨率达不到观测要求。电子显微镜(electron microscopy)被广泛用于表征多种生物样品。透射电子显微镜(transmission electron microscopy,TEM)是证明EVs存在的首选,常用的染色方法是负染,大多用乙酸氧铀或磷钨酸进行染色。TEM不仅能表征EVs的大小、形状和外观,还能识别样品中是否存在非EVs结构(如鞭毛和蛋白聚合物),经典的EVs呈现茶托状结构[23–24]。冷冻透射电子显微镜(cryo-transmission electron microscopy,cryo-TEM)也经常被用于表征EVs,样品首先被迅速冷冻,再用低温透射电镜对样品进行成像,不需要添加任何重金属或固定剂,相比TEM能更好地展示EVs的原本结构[25–26]。
2.2.2 EVs颗粒表征EVs颗粒表征通常包括粒径分布和颗粒浓度。动态光散射(dynamic light scattering)能测量EVs等纳米级颗粒物的粒径分布,但无法计算样品的颗粒浓度[27]。纳米颗粒追踪分析(nanoparticle tracking analysis)是常使用的表征颗粒大小和数量的方法之一,这种方法利用光散射和布朗运动的原理来确定纳米颗粒的大小和数量[28]。纳米流式检测装置(nano-flow cytometer)是近年新研发的装置,通过对单个纳米颗粒散射光强度的直接检测,获得纳米颗粒的粒径分布和粒子浓度,其检测限低,具有高灵敏、高选择性和高通量的特点[29]。
3 影响EVs分泌的因素EVs的产生受生物体本身以及所处环境的影响,表 2总结了能显著影响细菌分泌EVs的几种因素,包括pH、氧气含量、营养水平和抗生素等。尽管常态下细菌也会分泌EVs,当遭遇胁迫时,细菌会通过改变EVs的分泌数量,进行适应性调节,以应对胁迫,维持细菌生存。
| Impact factors | Bacteria | Results | References |
| pH | Salmonella enterica | An environmental change induced greater OMVs production and increased OMVs diameter | [16] |
| Oxygen | Pseudomonas aeruginosa | Under denitrifying condition, P. aeruginosa produced a considerable amount of MVs | [18] |
| Nutrition | Pseudomonas putida, Helicobacter pylori, Mycobacterium tuberculosis | Nutrient levels affected the production of EVs and more EVs produced by bacteria under iron limitation | [30–32] |
| Temperature | Pseudomonas putida | The amount of EVs secreted by the bacteria increased after exposure to 55 ℃ | [33] |
| Antibiotics stress | Staphylococcus aureus, Escherichia coli | The production of EVs was increased under antibiotic stressed conditions | [34–35] |
4 细菌EVs的生物学功能
EVs是纳米级颗粒,能携带蛋白质、毒素(toxin)、肽聚糖(peptidoglycan)、脂多糖(lipopolysaccharides,LPSs)和遗传物质(DNA、RNA)[36]。EVs内含物中的核酸组分包括质粒、染色体、甚至噬菌体DNA,长度一般在几百bp至几百kb[37–38]。包含不同内含物的EVs具有不同的功能,包括:结合噬菌体,失活抗生素,保护宿主细菌,向人体细胞传递毒力因子,影响人体健康,传递毒性物质,捕食竞争菌,介导水平基因转移,介导胞外电子转移,介导营养传递和促进生物膜形成等(图 2)。
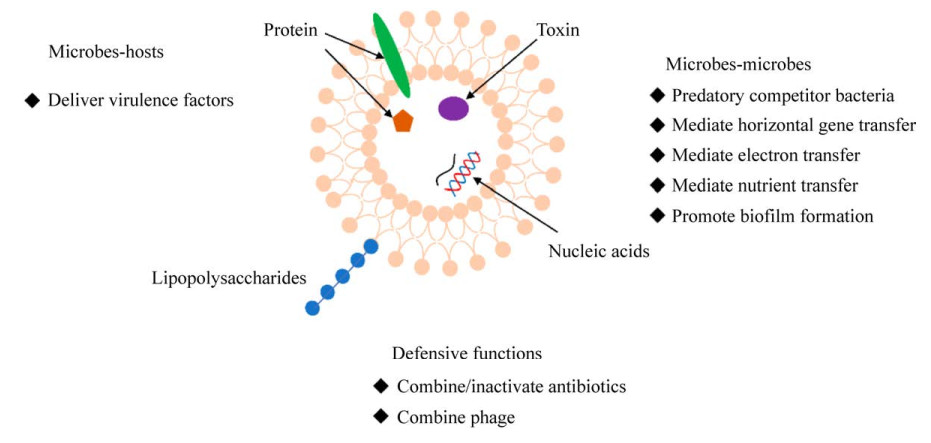
|
| 图 2 胞外囊泡的不同生物学功能 Figure 2 The diverse biological functions of extracellular vesicles. |
4.1 细菌EVs的防御功能 4.1.1 拮抗抗菌物质
在抗生素等抑菌物质胁迫下,分泌EVs成为细菌的一种防御机制。EVs可以结合抗生素等抗菌物质。研究发现,从金黄色葡萄球菌(S. aureus)培养液中提取的EVs可以与达托霉素(daptomycin,一种膜靶向抗生素)结合,从而保护细菌免受抗生素的攻击,抗生素诱导产生的EVs起到了“诱饵”的作用,有助于细菌的生存[39]。Manning等发现多粘菌素B和粘菌素(多肽类抗生素)会诱导大肠杆菌释放EVs,EVs随后通过吸附抗菌肽,从而消除抗菌肽对细菌的杀伤作用[40],抗菌肽可以结合LPS的脂质A区域,这种吸附很可能是通过与EVs携带的LPS结合而发生的[41–42]。以上是目前已报道的能与EVs结合的抗菌物质,脂双分子层颗粒结构赋予了EVs结合脂溶性物质的能力,未来随着研究的进一步深入,EVs能结合的抗菌物质清单会不断延长。
以上研究是基于实验室培养体系,也有报道在现实环境中,多粘菌素处理会诱导污水细菌群落产生大量的EVs,推测这些EVs能降低水中的抗生素浓度[43]。
除了结合抗生素,EVs也可携带抗生素水解酶,降解抗生素。β-内酰胺酶(β-lactamase)是一种能使β-内酰胺类抗生素失活的酶。在氨苄西林胁迫下,金黄色葡萄球菌(S. aureus)释放的EVs中含有大量能够降解β-内酰胺类抗生素的蛋白酶。功能学实验显示,抗生素与EVs共孵育后,抗生素浓度显著降低,说明EVs能够降解β-内酰胺类抗生素[34]。EVs还具有跨物种传递降解酶的作用。由拟杆菌属(Bacteroides spp.)分泌的含有β-内酰胺酶的EVs,能够水解β内酰胺类抗生素,保护共生细菌和肠道病原菌存活[44]。耐甲氧西林金黄色葡萄球菌(methicillin- resistant Staphylococcus aureus,MRSA)产生的EVs可以跨属保护细菌免受氨苄西林的抑制[34]。目前的研究聚焦于β-内酰胺酶,EVs是否包含其他类型的抗生素水解酶或修饰失活酶尚未见报道。鉴于EVs可携带多种类型的蛋白质,进一步筛选其他类型的抗生素水解酶或失活蛋白酶将是一项非常有意义的研究。
4.1.2 拮抗噬菌体由于EVs包含细菌膜的成分,如噬菌体能够识别宿主的膜表面蛋白,因此EVs还可以在保护细胞免受噬菌体的感染中发挥作用。透射电镜下能够观察到大量PHM-2噬菌体与原绿球藻的EVs结合,EVs附着的噬菌体柄变短,衣壳染色密度改变,表明噬菌体已将DNA注入EVs中[24]。Manning等通过测定噬菌斑形成单位数,发现T4噬菌体和EVs共孵育后,活性噬菌体显著减少约90%[40],EVs通过与T4噬菌体发生不可逆结合,显著降低T4噬菌体对细菌的感染。
4.2 EVs传递毒力因子EVs由磷脂双分子层膜包被,其独特的结构对内部的物质形成保护。EVs的特性使它们能够介导细胞毒素、毒力因子和各种其他生物活性分子的转移[45–46],并发挥信号通讯功能[38]。EVs可以跨界传递,铜绿假单胞菌(P. aeruginosa)分泌的EVs可输送小RNA到人体呼吸道上皮细胞,抑制先天免疫反应[47]。EVs的通讯是双向的,人体呼吸道上皮细胞分泌的EVs可传递miRNA let-7b-5p至铜绿假单胞菌,进而调节细菌对抗生素的敏感性,并降低细菌形成生物膜的能力[48]。EVs可以在细胞之间传递信号,我们研究发现塑化剂处理的人体肺上皮细胞可以分泌EVs,在细胞之间传递毒性信号[6, 49]。
EVs通过充当毒素进入宿主细胞的载体而促进细菌的致病性。EVs介导的毒性传递比纯毒素输送更有优势,而且通常更有效,从大肠杆菌中提取到的含有致孔细胞毒素ClyA的EVs,与等量纯化的ClyA相比,其对红细胞的溶解活性提高了10倍[50]。
EVs可以与人宿主细胞相互作用,它们可以传递外膜(outer membrane,OM)成分或其他物质,激活人体免疫系统[51–52],产生毒性[51]。产肠毒素大肠杆菌(enterotoxigenic Escherichia coli,ETEC)是发展中国家儿童腹泻的主要原因[53]。不耐热肠毒素(heat-labile enterotoxin,LT)是大肠杆菌产生的主要毒力因子之一,LT毒素位于EVs的内部和外部,并具有生物活性。研究表明,ETEC分泌的EVs可作为特异性靶向运输工具,介导活性LT进入小鼠肾上腺细胞,并使细胞发生形变[54],也能够破坏肠道上皮的电解质平衡[55–56]。细胞致死性膨胀毒素(cytolethal distending toxin,CDT)是空肠弯曲菌(Campylobacter jejuni)的主要毒力因子之一。研究表明,空肠弯曲菌通过EVs的释放,将所含CDT毒素亚基(CdtA、CdtB和CdtC)传递到周围环境,能够对人肠细胞系发挥CDT典型的细胞膨胀作用[57]。具核梭杆菌(Fusobacterium nucleatum)分泌的EVs携带多种毒力因子,促进巨噬细胞的M1极化,并通过激活FADD- RIPK1-caspase 3相关信号通路促进肠道上皮细胞程序性细胞坏死,从而破坏肠道上皮屏障[58]。铜绿假单胞菌分泌的EVs可通过与宿主细胞膜上的脂质体结合,同时远距离传递多种毒力因子,包括β-内酰胺酶、碱性磷酸酶(alkaline phosphatase)、溶血磷脂酶C和Cif等,并以协调的方式直接进入宿主细胞的细胞质[59]。鲍曼不动杆菌(Acinetobacter baumannii)产生的EVs包含OmpAAb蛋白(毒力因子),能够诱导宿主细胞(肺上皮细胞A549)线粒体碎裂和细胞毒性[60]。表 3列出了EVs携带多种毒力因子,但细菌如何通过EVs作用于细胞,进而影响人体健康,仍需进一步研究。
| Virulence factors | Bacteria | References |
| ClyA | Escherichia coli and enterobacteria | [50] |
| Heat-labile enterotoxin | Enterotoxigenic Escherichia coli | [54–56] |
| Cytolethal distending toxin (CdtA, CdtB and CdtC proteins) | Campylobacter jejuni | [57] |
| β-lactamase, alkaline phosphatase, hemolytic phospholipase C, and Cif | Pseudomonas aeruginosa | [59] |
| OmpAAb | Acinetobacter baumannii | [60] |
4.3 EVs在微生物群落中的互作 4.3.1 捕食竞争菌
宿主菌可通过分泌EVs,杀死竞争细菌[61]。铜绿假单胞菌分泌的EVs通过递送胞壁质水解酶,降解肽聚糖以杀死革兰氏阴性菌和革兰氏阳性菌[62]。来自金黄色葡萄球菌的EVs,富含N-乙酰胞壁酰-L-丙氨酸酰胺酶,能够通过降解肽聚糖来杀死邻近细菌[63]。溶菌属细菌分泌含有溶菌内肽酶L5的EVs,可杀死其他竞争性革兰氏阴性菌[64]。EVs携带多种内含物质,包括多种水解酶,宿主菌如何通过EVs杀死竞争菌,竞争菌如何响应这一外来压力,需要进一步研究。
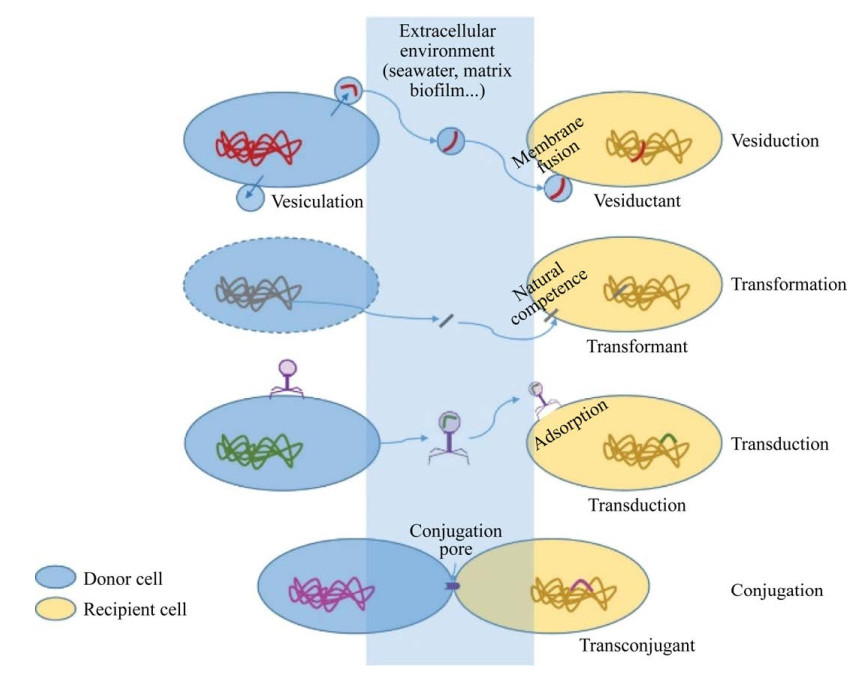
4.3.2 介导水平基因转移抗生素抗性基因(antibiotic resistance genes,ARGs)已成为一种新型环境污染物[65],不同于常规污染物,它可以在环境中持久性残留、通过增殖以及水平转移在环境中扩散。一旦ARGs转移至致病菌,就会降低抗生素的治疗效率,最终对人类健康构成严重威胁[66]。ARGs可通过垂直(基因突变)和水平转移传递,其中,水平基因转移(horizontal gene transfer,HGT)是ARGs传播扩散的主要方式,已知的HGT方式主要是接合、转化与转导。每种HGT在环境中的实现条件不同,受不同因素的限制[67]。接合需要在供体和受体细胞之间通过接合毛进行物理接触,遗传物质通过接合毛被转移。通常,接合只限于作为供体和受体的细菌细胞[68];转化是从环境中吸收外源性DNA,该方式在古细菌和细菌中都有报道[69–70];转导是将外源宿主遗传物质整合到噬菌体基因组中,通过噬菌体捕食来传递遗传物质的过程,这种现象在细菌和古细菌中都曾被发现[71–72]。学者们一直在不断寻找新的HGT方式。细菌分泌的纳米尺寸大小的基因转移物质(gene transfer agents,GTAs)受到关注,研究发现,海水中GTAs介导的转移频率比以往估计的HGT转移频率高多个数量级[73],而这种纳米级的物质可能是EVs。Soler等提出囊泡介导(vesiduction)的水平基因转移是第4种HGT方式[74] (图 3)。
EVs包裹并传递质粒和染色体DNA等遗传物质,可以以浓缩的方式将核酸在物种内,甚至跨物种传递扩散,其在细菌-细菌种间和种内的交流作用中发挥着重要作用[75]。鲍曼不动杆菌释放的EVs在种内传递碳青霉烯酶基因[76]。β-内酰胺类基因也可通过EVs在种内外转移,从而传递抗性,传递效率为10–5–10–6[77]。表 4总结了近年来EVs介导基因在种间和种内转移的实例。从表中可看出,EVs介导的基因水平转移研究中,主要是质粒携带的β-内酰胺类基因,此外,转移频率基于表型实验,即共孵育后的受体菌获得并表达抗性。其他功能基因能否通过EVs介导转移,也需进一步探索。与其他3种已知的水平基因转移效率相比,EVs介导的水平基因转移效率是怎样的,未来需进一步研究。
| Genes/Plasmids | Donors | Recipients | Transformation frequency | References |
| β-lactamase gene | Acinetobacter baylyi JV26 | Escherichia coli DH5α | 3×10–8 | [46] |
| blaOXA-24 | Acinetobacter baumannii AbH12O-A2, AbH12O-CU3 | Acinetobacter baumannii ATCC 17978 | – | [76] |
| blaNDM-1 aac(6′)-Ib-cr genes | Acinetobacter baumannii A_115 | Acinetobacter baumannii ATCC 19606 Escherichia coli JM109 |
10–5–10–6 | [77] |
| fimA gene | Porphyromonas gingivalis 49417 | Porphyromonas gingivalis 33277 | 1.9×10–7 | [78] |
| plasmid pBBR1MCS-1 | Buttiauxella agrestis CUETM77-167/pBBR1MCS-1 | Buttiauxella agrestis | – | [79] |
| blaCTX-M-15 blaTEM-1 | Escherichia coli 104:H4 | Enterobacteriaceae | 7.9×10–8–2.8×10–7 | [80] |
| gfp gene | Escherichia coli O157:H7 | Escherichia coli JM109 | 3×10–10 | [81] |
4.3.3 介导营养物质传递
EVs能够为细菌提供营养。原绿球藻(Prochlorococcus)是一种分布广、数量多的海洋蓝细菌,能够释放纳米级的EVs。研究表明,原绿球藻来源的EVs可以作为异养生物(交替单胞菌和盐单胞菌)的有机碳源[24]。EVs包含结合铁以及帮助获得铁的因子(如铁载体)。在铁离子限制情况下,结核分枝杆菌(Mycobacterium tuberculosis)分泌的EVs含有分枝杆菌素,分枝杆菌素可作为铁供体并支持缺铁分枝杆菌的生长[30]。蛋白组学揭示脑膜炎奈瑟菌(Neisseria meningitidis)分泌的EVs携带用于铁结合和吸收的蛋白质[82]。
4.3.4 介导电子传递EVs还能介导胞外电子的转移。硫还原地杆菌(Geobacter sulfurreducens)释放EVs可促进电子从胞外转移到阳极,地杆菌属(Geobacter spp.)释放的EVs不仅促进胞外电子转移,而且还赋予非产电菌产电能力[83]。
4.3.5 促进生物膜的形成生物膜被认为是环境中微生物的主要存在形式。生物膜能够保护微生物生长,抵抗抗菌物质的干扰[84]。生物膜中细菌之间的信号通讯频繁,而EVs作为通讯的重要载体,在生物膜的形成过程中发挥重要的作用。EVs中的货物可以为生物膜提供基底。研究发现,变形链球菌(Streptococcus mutans)释放的EVs包含eDNA和粘附蛋白,不仅有利于自身生物膜的形成[85],而且增强了白色念珠菌(Candida albicans)生物膜的形成[86]。南极地区希瓦氏菌(S. vesiculosa M7T)分泌的EVs携带ATP,能够促进生物膜的形成[87]。不同的EVs组分对生物膜的形成和维持是必不可少的,但由于EVs组分的复杂性,各组分的作用还需进一步阐明。
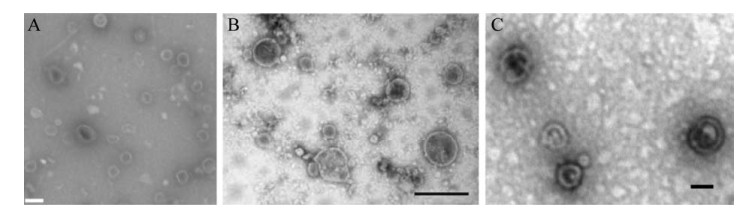
5 胞外囊泡在环境中的分布目前针对环境中的EVs,已发表的研究集中在污水、海水、灰尘和温泉。图 4为环境样品来源的EVs透射电镜图,细菌可分泌EVs到环境中已被证实。这些EVs发挥着不同的功能,表 5展示了环境样品来源EVs的信息和功能。
| Environmental medium | Mean size/nm | Concentration | Function | References |
| Seawater | – | 3.0×105–6.0×106 particles/mL | Prochlorococcus vesicles can support the growth of heterotrophic bacterial cultures, which implicates these structures in marine carbon flux | [24] |
| Wastewater | 125.0 | 2.0×106 particles/mL | Vesicles could have a more active role in the context of transmission of antibiotic resistances | [43] |
| Dust | 129.6±4.5 | (1.1±0.6)×1011 particles/g | Indoor dust EVs are potential promoting agents of cancer lung metastasis | [88] |
| Spring | – | – | EVs play an important role in horizontal gene transfer and nutrient cycling in extreme environments | [90] |
| Dust | – | – | Indoor dust EV, especially derived from Gram-negative bacteria, is a possible causative agent of neutrophilic airway diseases | [91] |
城市污水处理厂是城市的“肠道”,是一个巨大的抗生素抗性基因库。Schooling等最早通过透射电镜在废水生物膜中观察到EVs[89],多粘菌素处理污水厂的出水样品后,出水的滤液中类似EVs结构组分显著增加,而这类组分含有ARGs,该组分可能参与污水中ARGs的转移[44]。污水中存在的EVs能否在污水处理过程中保持稳定性,其能否被降解,目前是未知的。小尺寸的EVs作为ARGs的移动储存库,在污水中EVs携带ARGs的丰度是未知的,了解这一现象,将进一步丰富我们对ARGs的认识。
Biller等发现EVs广泛存在于海水中。研究发现,原绿球藻产生的EVs可能和海洋碳通量相关,同时研究发现原绿球藻EVs具有一定的稳定性,在21 ℃的无菌海水中可稳定存在两周[24]。温泉中古菌EVs以内吞体运输分拣复合物(the endosomal sorting complex required for transport,ESCRT)依赖性方式产生,并促进极端环境中的基因转移和营养循环[90]。
室内污染物已被列为影响公众健康的最重要环境问题之一,室内污染物与人体健康密切相关。持续暴露于室内污染物会引起呼吸道疾病,对人类健康构成潜在威胁。在室内污染物中,室内灰尘含有EVs等多种物质。室内灰尘中的EVs,尤其是革兰氏阴性菌产生的EVs,可诱导肺部炎症[91]。Dinh等发现室内灰尘中存在的EVs主要来源于细菌,能够以剂量依赖的方式增强小鼠黑色素瘤肺转移[88]。
EVs在环境中广泛存在,说明了:(1) 环境中,细菌不断地分泌EVs;(2) EVs可以抵御不良环境胁迫,具有较高的稳定性;(3) 细菌通过EVs适应环境胁迫。研究发现,通过基因工程使有机磷水解酶连接到EVs上,形成基于EVs的生物催化剂,这些生物催化剂对有机磷(含氧磷)表现出较强的降解率,这表明它们在有机磷农药的降解中具有潜在的用途[92],在土壤有机磷农药污染修复方面具有潜在价值。
针对环境中EVs的研究,主要存在以下困难:首先是环境样品处理体积较大,过程比较烦琐;其次,环境样品中物质比较复杂,存在非囊泡物质干扰,比如噬菌体。由于病毒与EVs尺寸相似,目前的技术手段无法将非囊泡物质(主要是病毒)与EVs分离。针对环境样品,如何排除病毒的干扰需要进一步研究。前期,我们从环境样品中提取EVs,鉴定并发现EVs在环境中广泛存在,通过宏基因组研究其来源和内含的功能基因,相关的测序数据已上传至科学数据银行数据库[93]。未来,基于组学技术深入分析EVs在环境中的分布、来源组成、内含物组成和功能,将为我们了解细菌与环境的合作提供新的思路。
6 结论与展望本文总结了近年来EVs在保护细菌生存、介导基因水平转移、传递毒力因子和细胞间通讯等方面的功能,阐述影响细菌分泌EVs的因素,以及EVs在环境中的分布特征。作者认为应在以下方面展开进一步探究。
(1) EVs的分泌是自然界中细胞生命活动普遍存在的一种现象,其产生机制还有待深入研究。
(2) 细菌分泌的EVs能够使抗生素失效,从而保护宿主菌或共生菌的生存。目前已知的作用方式,包括水解抗生素和吸附抗生素,EVs如何使得抗生素失效,还需进一步研究。除此之外,当前研究主要针对β-内酰胺类抗生素,鉴于EVs可携带多种类型的蛋白质,今后可进一步探索EVs是否包含其他类型的抗生素水解酶或修饰失活酶类。
(3) 细菌通过分泌EVs响应外界刺激(如物理环境和环境污染物),EVs的功能解析有助于揭示细菌响应环境胁迫的机制。
(4) 由于对EVs在环境介质中的存在状况、来源及其功能的了解较少,有必要进一步开展相关研究,更好地了解EVs在环境中发挥的功能。
(5) 作为一种新的水平基因转移方式,EVs介导的水平基因转移还有待进一步研究,所研究的基因包括但不局限于抗生素抗性基因,与其他3种水平基因转移方式相比,EVs介导的转移效率也需进一步研究。
| [1] | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science, 2020, 367(Feb. 7 TN. 6478): 640. |
| [2] | Gill S, Catchpole R, Forterre P. Extracellular membrane vesicles in the three domains of life and beyond. FEMS Microbiology Reviews, 2019, 43(3): 273-303. DOI:10.1093/femsre/fuy042 |
| [3] | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. Journal of Extracellular Vesicles, 2018, 7(1): 1535750. DOI:10.1080/20013078.2018.1535750 |
| [4] | Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature Reviews Immunology, 2009, 9(8): 581-593. DOI:10.1038/nri2567 |
| [5] | Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, Zambirinis CP, Rodrigues G, Molina H, Heissel S, Mark MT, Steiner L, Benito-Martin A, Lucotti S, Di Giannatale A, Offer K, Nakajima M, Williams C, Lyden D. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell, 2020, 182(4): 1044-1061. DOI:10.1016/j.cell.2020.07.009 |
| [6] | Qin YF, Zhang J, Avellán-Llaguno RD, Zhang X, Huang QS. DEHP-elicited small extracellular vesicles miR-26a-5p promoted metastasis in nearby normal A549 cells. Environmental Pollution, 2021, 272: 116005. DOI:10.1016/j.envpol.2020.116005 |
| [7] | Takasugi M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell, 2018, 17(2): e12734. DOI:10.1111/acel.12734 |
| [8] | Work E, Knox KW, Vesk M. The chemistry and electron microscopy of an extracellular lipopolysaccharide from Escherichia coli. Annals of the New York Academy of Sciences, 1966, 133(2): 438-449. |
| [9] | Marsollier L, Brodin P, Jackson M, Korduláková J, Tafelmeyer P, Carbonnelle E, Aubry J, Milon G, Legras P, André JP, Leroy C, Cottin J, Guillou ML, Reysset G, Cole ST. Impact of Mycobacterium ulcerans biofilm on transmissibility to ecological niches and Buruli ulcer pathogenesis. PLoS Pathogens, 2007, 3(5): e62. DOI:10.1371/journal.ppat.0030062 |
| [10] | Toyofuku M, Nomura N, Eberl L. Types and origins of bacterial membrane vesicles. Nature Reviews Microbiology, 2019, 17(1): 13-24. DOI:10.1038/s41579-018-0112-2 |
| [11] | Roier S, Zingl FG, Cakar F, Schild S. Bacterial outer membrane vesicle biogenesis: a new mechanism and its implications. Microbial Cell, 2016, 3(6): 257-259. DOI:10.15698/mic2016.06.508 |
| [12] | Pérez-Cruz C, Carrión O, Delgado L, Martinez G, López-Iglesias C, Mercade E. New type of outer membrane vesicle produced by the Gram-negative bacterium Shewanella vesiculosa M7T: implications for DNA content. Applied and Environmental Microbiology, 2013, 79(6): 1874-1881. DOI:10.1128/AEM.03657-12 |
| [13] | Turnbull L, Toyofuku M, Hynen AL, Kurosawa M, Pessi G, Petty NK, Osvath SR, Cárcamo-Oyarce G, Gloag ES, Shimoni R, Omasits U, Ito S, Yap X, Monahan LG, Cavaliere R, Ahrens CH, Charles IG, Nomura N, Eberl L, Whitchurch CB. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nature Communications, 2016, 7: 11220. DOI:10.1038/ncomms11220 |
| [14] | Toyofuku M, Cárcamo-Oyarce G, Yamamoto T, Eisenstein F, Hsiao CC, Kurosawa M, Gademann K, Pilhofer M, Nomura N, Eberl L. Prophage-triggered membrane vesicle formation through peptidoglycan damage in Bacillus subtilis. Nature Communications, 2017, 8: 481. DOI:10.1038/s41467-017-00492-w |
| [15] | Shingaki R, Kasahara Y, Inoue T, Kokeguchi S, Fukui K. Chromosome DNA fragmentation and excretion caused by defective prophage gene expression in the early-exponential-phase culture of Bacillus subtilis. Canadian Journal of Microbiology, 2003, 49(5): 313-325. DOI:10.1139/w03-041 |
| [16] | Bonnington KE, Kuehn MJ. Outer membrane vesicle production facilitates LPS remodeling and outer membrane maintenance in Salmonella during environmental transitions. mBio, 2016, 7(5): e01532-e01516. |
| [17] | Hong J, Dauros-Singorenko P, Whitcombe A, Payne L, Blenkiron C, Phillips A, Swift S. Analysis of the Escherichia coli extracellular vesicle proteome identifies markers of purity and culture conditions. Journal of Extracellular Vesicles, 2019, 8(1): 1632099. DOI:10.1080/20013078.2019.1632099 |
| [18] | Toyofuku M, Zhou SM, Sawada I, Takaya N, Uchiyama H, Nomura N. Membrane vesicle formation is associated with pyocin production under denitrifying conditions in Pseudomonas aeruginosa PAO1. Environmental Microbiology, 2014, 16(9): 2927-2938. DOI:10.1111/1462-2920.12260 |
| [19] | Collins SM, Nice JB, Chang EH, Brown AC. Size exclusion chromatography to analyze bacterial outer membrane vesicle heterogeneity. Journal of Visualized Experiments, 2021, 169: e62429. |
| [20] | Klimentová J, Stulík J. Methods of isolation and purification of outer membrane vesicles from Gram-negative bacteria. Microbiological Research, 2015, 170: 1-9. DOI:10.1016/j.micres.2014.09.006 |
| [21] | Oishi S, Miyashita M, Kiso A, Kikuchi Y, Ueda O, Hirai K, Shibata Y, Fujimura S. Cellular locations of proteinases and association with vesicles in Porphyromonas gingivalis. European Journal of Medical Research, 2010, 15(9): 397-402. DOI:10.1186/2047-783X-15-9-397 |
| [22] | Alves NJ, Turner KB, Di Vito KA, Daniele MA, Walper SA. Affinity purification of bacterial outer membrane vesicles (OMVs) utilizing a His-tag mutant. Research in Microbiology, 2017, 168(2): 139-146. DOI:10.1016/j.resmic.2016.10.001 |
| [23] | Tulkens J, De Wever O, Hendrix A. Analyzing bacterial extracellular vesicles in human body fluids by orthogonal biophysical separation and biochemical characterization. Nature Protocols, 2020, 15(1): 40-67. DOI:10.1038/s41596-019-0236-5 |
| [24] | Biller SJ, Schubotz F, Roggensack SE, Thompson AW, Summons RE, Chisholm SW. Bacterial vesicles in marine ecosystems. Science, 2014, 343(6167): 183-186. DOI:10.1126/science.1243457 |
| [25] | Emelyanov A, Shtam T, Kamyshinsky R, Garaeva L, Verlov N, Miliukhina I, Kudrevatykh A, Gavrilov G, Zabrodskaya Y, Pchelina S, Konevega A. Cryo-electron microscopy of extracellular vesicles from cerebrospinal fluid. PLoS One, 2020, 15(1): e0227949. DOI:10.1371/journal.pone.0227949 |
| [26] | Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The methods of choice for extracellular vesicles (EVs) characterization. International Journal of Molecular Sciences, 2017, 18(6): 1153. DOI:10.3390/ijms18061153 |
| [27] | Shpacovitch V, Hergenröder R. Optical and surface plasmonic approaches to characterize extracellular vesicles.A review. Analytica Chimica Acta, 2018, 1005: 1-15. DOI:10.1016/j.aca.2017.11.066 |
| [28] | Gardiner C, Vizio DD, Sahoo S, Théry C, Witwer KW, Wauben M, Hill AF. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. Journal of Extracellular Vesicles, 2016, 5(1): 32945. DOI:10.3402/jev.v5.32945 |
| [29] | Tian Y, Gong MF, Hu YY, Liu HS, Zhang WQ, Zhang MM, Hu XX, Aubert D, Zhu SB, Wu LN, Yan XM. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. Journal of Extracellular Vesicles, 2020, 9(1): 1697028. DOI:10.1080/20013078.2019.1697028 |
| [30] | Prados-Rosales R, Weinrick BC, Piqué DG, Jacobs WR Jr, Casadevall A, Rodriguez GM. Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. Journal of Bacteriology, 2014, 196(6): 1250-1256. DOI:10.1128/JB.01090-13 |
| [31] | Choi CW, Park EC, Yun SH, Lee SY, Lee YG, Hong Y, Park KR, Kim SH, Kim GH, Kim SI. Proteomic characterization of the outer membrane vesicle of Pseudomonas putida KT2440. Journal of Proteome Research, 2014, 13(10): 4298-4309. DOI:10.1021/pr500411d |
| [32] | Keenan JI, Davis KA, Beaugie CR, McGovern JJ, Moran AP. Alterations in Helicobacter pylori outer membrane and outer membrane vesicle-associated lipopolysaccharides under iron-limiting growth conditions. Innate Immunity, 2008, 14(5): 279-290. DOI:10.1177/1753425908096857 |
| [33] | Baumgarten T, Sperling S, Seifert J, Von Bergen M, Steiniger F, Wick LY, Heipieper HJ. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Applied and Environmental Microbiology, 2012, 78(17): 6217-6224. DOI:10.1128/AEM.01525-12 |
| [34] | Kim SW, Seo JS, Park SB, Lee AR, Lee JS, Jung JW, Chun JH, Lazarte JMS, Kim J, Kim JH, Song JW, Franco C, Zhang W, Ha MW, Paek SM, Jung M, Jung TS. Significant increase in the secretion of extracellular vesicles and antibiotics resistance from methicillin-resistant Staphylococcus aureus induced by ampicillin stress. Scientific Reports, 2020, 10: 21066. DOI:10.1038/s41598-020-78121-8 |
| [35] | Bos J, Cisneros LH, Mazel D. Real-time tracking of bacterial membrane vesicles reveals enhanced membrane traffic upon antibiotic exposure. Science Advances, 2021, 7(4): eabd1033. DOI:10.1126/sciadv.abd1033 |
| [36] | Uddin MJ, Dawan J, Jeon G, Yu T, He XL, Ahn J. The role of bacterial membrane vesicles in the dissemination of antibiotic resistance and as promising carriers for therapeutic agent delivery. Microorganisms, 2020, 8(5): 670. DOI:10.3390/microorganisms8050670 |
| [37] | Orench-Rivera N, Kuehn MJ. Environmentally controlled bacterial vesicle-mediated export. Cellular Microbiology, 2016, 18(11): 1525-1536. DOI:10.1111/cmi.12676 |
| [38] | Domingues S, Nielsen KM. Membrane vesicles and horizontal gene transfer in prokaryotes. Current Opinion in Microbiology, 2017, 38: 16-21. DOI:10.1016/j.mib.2017.03.012 |
| [39] | Andreoni F, Toyofuku M, Menzi C, Kalawong R, Mairpady Shambat S, François P, Zinkernagel AS, Eberl L. Antibiotics stimulate formation of vesicles in Staphylococcus aureus in both phage-dependent and -independent fashions and via different routes. Antimicrobial Agents and Chemotherapy, 2019, 63(2): e01439-18. |
| [40] | Manning AJ, Kuehn MJ. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiology, 2011, 11: 258. DOI:10.1186/1471-2180-11-258 |
| [41] | Steimle A, Autenrieth IB, Frick JS. Structure and function: lipid A modifications in commensals and pathogens. International Journal of Medical Microbiology, 2016, 306(5): 290-301. DOI:10.1016/j.ijmm.2016.03.001 |
| [42] | Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria?. Nature Reviews Microbiology, 2005, 3(3): 238-250. DOI:10.1038/nrmicro1098 |
| [43] | Maestre-Carballa L, Lluesma Gomez M, Angla Navarro A, Garcia-Heredia I, Martinez-Hernandez F, Martinez-Garcia M. Insights into the antibiotic resistance dissemination in a wastewater effluent microbiome: bacteria, viruses and vesicles matter. Environmental Microbiology, 2019, 21(12): 4582-4596. DOI:10.1111/1462-2920.14758 |
| [44] | Stentz R, Horn N, Cross K, Salt L, Brearley C, Livermore DM, Carding SR. Cephalosporinases associated with outer membrane vesicles released by Bacteroides spp.protect gut pathogens and commensals against β-lactam antibiotics. Journal of Antimicrobial Chemotherapy, 2015, 70(3): 701-709. |
| [45] | Furuta N, Takeuchi H, Amano A. Entry of Porphyromonas gingivalis outer membrane vesicles into epithelial cells causes cellular functional impairment. Infection and Immunity, 2009, 77(11): 4761-4770. DOI:10.1128/IAI.00841-09 |
| [46] | Fulsundar S, Harms K, Flaten GE, Johnsen PJ, Chopade BA, Nielsen KM. Gene transfer potential of outer membrane vesicles of Acinetobacter baylyi and effects of stress on vesiculation. Applied and Environmental Microbiology, 2014, 80(11): 3469-3483. DOI:10.1128/AEM.04248-13 |
| [47] | Koeppen K, Hampton TH, Jarek M, Scharfe M, Gerber SA, Mielcarz DW, Demers EG, Dolben EL, Hammond JH, Hogan DA, Stanton BA. A novel mechanism of host-pathogen interaction through sRNA in bacterial outer membrane vesicles. PLoS Pathogens, 2016, 12(6): e1005672. DOI:10.1371/journal.ppat.1005672 |
| [48] | Koeppen K, Nymon A, Barnaby R, Bashor L, Li ZY, Hampton TH, Liefeld AE, Kolling FW, LaCroix IS, Gerber SA, Hogan DA, Kasetty S, Nadell CD, Stanton BA. Let-7b-5p in vesicles secreted by human airway cells reduces biofilm formation and increases antibiotic sensitivity of P. aeruginosa. PNAS, 2021, 118(28): e2105370118. DOI:10.1073/pnas.2105370118 |
| [49] | Qin YF, Long L, Huang QS. Extracellular vesicles in toxicological studies: key roles in communication between environmental stress and adverse outcomes. Journal of Applied Toxicology, 2020, 40(9): 1166-1182. DOI:10.1002/jat.3963 |
| [50] | Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, Jass J, Richter-Dahlfors A, Mizunoe Y, Uhlin BE. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell, 2003, 115(1): 25-35. DOI:10.1016/S0092-8674(03)00754-2 |
| [51] | Li ZT, Zhang RL, Bi XG, Xu L, Fan M, Xie D, Xian Y, Wang Y, Li XJ, Wu ZD, Zhang KX. Outer membrane vesicles isolated from two clinical Acinetobacter baumannii strains exhibit different toxicity and proteome characteristics. Microbial Pathogenesis, 2015, 81: 46-52. DOI:10.1016/j.micpath.2015.03.009 |
| [52] | Jun SH, Lee JH, Kim BR, Kim SI, Park TI, Lee JC, Lee YC. Acinetobacter baumannii outer membrane vesicles elicit a potent innate immune response via membrane proteins. PLoS One, 2013, 8(8): e71751. DOI:10.1371/journal.pone.0071751 |
| [53] | Fleckenstein JM, Kuhlmann FM. Enterotoxigenic Escherichia coli infections. Current Infectious Disease Reports, 2019, 21(3): 1-9. |
| [54] | Kesty NC, Mason KM, Reedy M, Miller SE, Kuehn MJ. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. The EMBO Journal, 2004, 23(23): 4538-4549. DOI:10.1038/sj.emboj.7600471 |
| [55] | Mirhoseini A, Amani J, Nazarian S. Review on pathogenicity mechanism of enterotoxigenic Escherichia coli and vaccines against it. Microbial Pathogenesis, 2018, 117: 162-169. DOI:10.1016/j.micpath.2018.02.032 |
| [56] | Horstman AL, Kuehn MJ. Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway. Journal of Biological Chemistry, 2002, 277(36): 32538-32545. DOI:10.1074/jbc.M203740200 |
| [57] | Lindmark B, Rompikuntal PK, Vaitkevicius K, Song TY, Mizunoe Y, Uhlin BE, Guerry P, Wai SN. Outer membrane vesicle-mediated release of cytolethal distending toxin (CDT) from Campylobacter jejuni. BMC Microbiology, 2009, 9(1): 1-10. DOI:10.1186/1471-2180-9-1 |
| [58] | Liu L, Liang LP, Yang CH, Zhou YL, Chen Y. Extracellular vesicles of Fusobacterium nucleatum compromise intestinal barrier through targeting RIPK1-mediated cell death pathway. Gut Microbes, 2021, 13(1): 1902718. DOI:10.1080/19490976.2021.1902718 |
| [59] | Bomberger JM, Maceachran DP, Coutermarsh BA, Ye SY, O'Toole GA, Stanton BA. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathogens, 2009, 5(4): e1000382. DOI:10.1371/journal.ppat.1000382 |
| [60] | Tiku V, Kofoed EM, Yan DH, Kang J, Xu M, Reichelt M, Dikic I, Tan MW. Outer membrane vesicles containing OmpA induce mitochondrial fragmentation to promote pathogenesis of Acinetobacter baumannii. Scientific Reports, 2021, 11: 618. DOI:10.1038/s41598-020-79966-9 |
| [61] | MacDonald IA, Kuehn MJ. Offense and defense: microbial membrane vesicles play both ways. Research in Microbiology, 2012, 163(9/10): 607-618. |
| [62] | Kadurugamuwa JL, Beveridge TJ. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other Gram-negative bacteria. Microbiology: Reading, England, 1999, 145(Pt 8): 2051-2060. |
| [63] | Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics, 2009, 9(24): 5425-5436. DOI:10.1002/pmic.200900338 |
| [64] | Vasilyeva NV, Tsfasman IM, Suzina NE, Stepnaya OA, Kulaev IS. Secretion of bacteriolytic endopeptidase L5 of Lysobacter sp.into the medium by means of outer membrane vesicles. The FEBS Journal, 2008, 275(15): 3827-3835. |
| [65] | Pruden A, Pei RT, Storteboom H, Carlson KH. Antibiotic resistance genes as emerging contaminants: studies in northern Colorado. Environmental Science & Technology, 2006, 40(23): 7445-7450. |
| [66] | Allen HK, Donato J, Wang HH, Cloud-Hansen KA, Davies J, Handelsman J. Call of the wild: antibiotic resistance genes in natural environments. Nature Reviews Microbiology, 2010, 8(4): 251-259. DOI:10.1038/nrmicro2312 |
| [67] | Nazarian P, Tran F, Boedicker JQ. Modeling multispecies gene flow dynamics reveals the unique roles of different horizontal gene transfer mechanisms. Frontiers in Microbiology, 2018, 9: 2978. DOI:10.3389/fmicb.2018.02978 |
| [68] | Norman A, Hansen LH, Sørensen SJ. Conjugative plasmids: vessels of the communal gene pool. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 2009, 364(1527): 2275-2289. DOI:10.1098/rstb.2009.0037 |
| [69] | Johnston C, Martin B, Fichant G, Polard P, Claverys JP. Bacterial transformation: distribution, shared mechanisms and divergent control. Nature Reviews Microbiology, 2014, 12(3): 181-196. DOI:10.1038/nrmicro3199 |
| [70] | Soucy SM, Huang JL, Gogarten JP. Horizontal gene transfer: building the web of life. Nature Reviews Genetics, 2015, 16(8): 472-482. DOI:10.1038/nrg3962 |
| [71] | Ross J, Topp E. Abundance of antibiotic resistance genes in bacteriophage following soil fertilization with dairy manure or municipal biosolids, and evidence for potential transduction. Applied and Environmental Microbiology, 2015, 81(22): 7905-7913. DOI:10.1128/AEM.02363-15 |
| [72] | Von Wintersdorff CJH, Penders J, Van Niekerk JM, Mills ND, Majumder S, Van Alphen LB, Savelkoul PHM, Wolffs PFG. Dissemination of antimicrobial resistance in microbial ecosystems through horizontal gene transfer. Frontiers in Microbiology, 2016, 7: 173. |
| [73] | Chiura HX, Kogure K, Hagemann S, Ellinger A, Velimirov B. Evidence for particle-induced horizontal gene transfer and serial transduction between bacteria. FEMS Microbiology Ecology, 2011, 76(3): 576-591. DOI:10.1111/j.1574-6941.2011.01077.x |
| [74] | Soler N, Forterre P. Vesiduction: the fourth way of HGT. Environmental Microbiology, 2020, 22(7): 2457-2460. DOI:10.1111/1462-2920.15056 |
| [75] | Jan AT. Outer membrane vesicles (OMVs) of Gram-negative bacteria: a perspective update. Frontiers in Microbiology, 2017, 8: 1053. DOI:10.3389/fmicb.2017.01053 |
| [76] | Rumbo C, Fernández-Moreira E, Merino M, Poza M, Mendez JA, Soares NC, Mosquera A, Chaves F, Bou G. Horizontal transfer of the OXA-24 carbapenemase gene via outer membrane vesicles: a new mechanism of dissemination of carbapenem resistance genes in Acinetobacter baumannii. Antimicrobial Agents and Chemotherapy, 2011, 55(7): 3084-3090. DOI:10.1128/AAC.00929-10 |
| [77] | Chatterjee S, Mondal A, Mitra S, Basu S. Acinetobacter baumannii transfers the blaNDM-1 gene via outer membrane vesicles. Journal of Antimicrobial Chemotherapy, 2017, 72(8): 2201-2207. DOI:10.1093/jac/dkx131 |
| [78] | Ho MH, Chen CH, Goodwin JS, Wang BY, Xie H. Functional advantages of Porphyromonas gingivalis vesicles. PLoS One, 2015, 10(4): e0123448. DOI:10.1371/journal.pone.0123448 |
| [79] | Tashiro Y, Hasegawa Y, Shintani M, Takaki K, Ohkuma M, Kimbara K, Futamata H. Interaction of bacterial membrane vesicles with specific species and their potential for delivery to target cells. Frontiers in Microbiology, 2017, 8: 571. |
| [80] | Bielaszewska M, Daniel O, Karch H, Mellmann A. Dissemination of the blaCTX-M-15 gene among Enterobacteriaceae via outer membrane vesicles. Journal of Antimicrobial Chemotherapy, 2020, 75(9): 2442-2451. DOI:10.1093/jac/dkaa214 |
| [81] | Yaron S, Kolling GL, Simon L, Matthews KR. Vesicle-mediated transfer of virulence genes from Escherichia coli O157: H7 to other enteric bacteria. Applied and Environmental Microbiology, 2000, 66(10): 4414-4420. DOI:10.1128/AEM.66.10.4414-4420.2000 |
| [82] | Lappann M, Otto A, Becher D, Vogel U. Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. Journal of Bacteriology, 2013, 195(19): 4425-4435. DOI:10.1128/JB.00625-13 |
| [83] | Liu X, Jing XY, Ye Y, Zhan J, Ye J, Zhou SG. Bacterial vesicles mediate extracellular electron transfer. Environmental Science & Technology Letters, 2020, 7(1): 27-34. |
| [84] | Kumar A, Alam A, Rani M, Ehtesham NZ, Hasnain SE. Biofilms: survival and defense strategy for pathogens. International Journal of Medical Microbiology, 2017, 307(8): 481-489. DOI:10.1016/j.ijmm.2017.09.016 |
| [85] | Cao YN, Zhou Y, Chen DR, Wu RX, Guo LH, Lin HC. Proteomic and metabolic characterization of membrane vesicles derived from Streptococcus mutans at different pH values. Applied Microbiology and Biotechnology, 2020, 104(22): 9733-9748. DOI:10.1007/s00253-020-10563-6 |
| [86] | Wu RX, Tao Y, Cao YN, Zhou Y, Lin HC. Streptococcus mutans membrane vesicles harboring glucosyltransferases augment Candida albicans biofilm development. Frontiers in Microbiology, 2020, 11: 581184. DOI:10.3389/fmicb.2020.581184 |
| [87] | Baeza N, Mercade E. Relationship between membrane vesicles, extracellular ATP and biofilm formation in Antarctic Gram-negative bacteria. Microbial Ecology, 2021, 81(3): 645-656. DOI:10.1007/s00248-020-01614-6 |
| [88] | Dinh NTH, Lee J, Lee J, Kim SS, Go G, Bae S, Jun YI, Yoon YJ, Roh TY, Gho YS. Indoor dust extracellular vesicles promote cancer lung metastasis by inducing tumour necrosis factor-Α. Journal of Extracellular Vesicles, 2020, 9(1): 1766821. DOI:10.1080/20013078.2020.1766821 |
| [89] | Schooling SR, Beveridge TJ. Membrane vesicles: an overlooked component of the matrices of biofilms. Journal of Bacteriology, 2006, 188(16): 5945-5957. DOI:10.1128/JB.00257-06 |
| [90] | Liu JF, Cvirkaite-Krupovic V, Commere PH, Yang YF, Zhou F, Forterre P, Shen YL, Krupovic M. Archaeal extracellular vesicles are produced in an ESCRT-dependent manner and promote gene transfer and nutrient cycling in extreme environments. The ISME Journal, 2021, 15(10): 2892-2905. DOI:10.1038/s41396-021-00984-0 |
| [91] | Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J, Jee YK, Zhu Z, Koh YY, Gho YS, Kim YK. Extracellular vesicles, especially derived from Gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. Clinical & Experimental Allergy, 2013, 43(4): 443-454. |
| [92] | Su FH, Tabañag IDF, Wu CY, Tsai SL. Decorating outer membrane vesicles with organophosphorus hydrolase and cellulose binding domain for organophosphate pesticide degradation. Chemical Engineering Journal, 2017, 308: 1-7. DOI:10.1016/j.cej.2016.09.045 |
| [93] | Huang HN, Zhu LT, Guo ZH, Huang QS. Raw metagenomic sequencing data of extracellular vesicles (EVs) and their habitats (V1). 2021. Science Data Bank. 2021-09-28. cstr: 31253.11. sciencedb. 01118;https://datapid.cn/31253.11.sciencedb.01118. |
 2022, Vol. 62
2022, Vol. 62