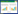中国科学院微生物研究所,中国微生物学会
文章信息
- 范敬阳, 王红越, 王泽铭, 王润博, 徐爽, 程安宁, 于景丽. 2022
- FAN Jingyang, WANG Hongyue, WANG Zeming, WANG Runbo, XU Shuang, CHENG Anning, YU Jingli.
- 锡林河流域潜在不产氧光合细菌陆向分异特征及影响因素
- Landward differentiation characteristics of anoxygenic photosynthetic bacterial populations and their influencing factors in Xilin River Basin
- 微生物学报, 62(6): 2353-2371
- Acta Microbiologica Sinica, 62(6): 2353-2371
-
文章历史
- 收稿日期:2022-03-31
- 修回日期:2022-05-08
- 网络出版日期:2022-05-19
2. 内蒙古自治区环境污染控制与废物资源化重点实验室, 内蒙古 呼和浩特 010021;
3. 内蒙古大学生态与环境学院, 内蒙古 呼和浩特 010021
2. Inner Mongolia Key Laboratory of Environmental Pollution Control & Waste Resource Reuse, Hohhot 010021, Inner Mongolia, China;
3. School of Ecology and Environment, Inner Mongolia University, Hohhot 010021, Inner Mongolia, China
不产氧光合细菌(anoxygenic photosynthetic/phototrophic bacteria,AnPB)作为海洋中主要的初级生产者是表征地球演化、生命起源和光合进化的标志性生物,能利用硫化物、单质硫、硫代硫酸盐、硫酸盐等作为电子供体进行不产氧光合作用[1–3]。AnPB只有一个反应中心,Ⅰ型以Fe-S为反应中心、Ⅱ型以苯醌为反应中心[3]。Ⅰ型包括来自绿菌门的绿色硫细菌(green sulfur bacteria,GSB)、厚壁菌门的Heliobacteria和酸杆菌门的微生物;Ⅱ型包括来自变形菌门的紫色硫细菌(purple sulfur bacteria,PSB)和紫色非硫细菌(purple non-sulfur bacteria,PNSB)、来自绿弯菌门的绿色非硫细菌(green non-sulfur bacteria,GNSB)和芽单胞菌门[1, 3]。多数PNSB (慢生根瘤菌科[4]和红环菌科[5])具有良好的固氮功能,可作为生物肥料的原材料;PNSB中的红环菌科具有硝酸盐还原和降解芳香族化合物等功能[6–7];丛毛单胞菌科具有生物除磷作用[8]。PSB是微生物席上的优势菌[9],包括胞内产硫的着色杆菌科和胞外产硫的外硫红螺菌科[10]。芽单胞菌科能改善土壤肥力状况促进植物生长[11]。干旱区生物结皮土壤中AnPB具有增加土壤碳汇、固氮和改善土壤条件等功能[12]。简言之,上述AnPB具有固碳、固氮、除磷、降解有机污染物等生态功能,属于环境友好型微生物。此外,GSB和PSB作为碳氮硫生物地球化学循环的主要驱动者在缺氧环境下是水华的主要贡献者[13]。
AnPB广泛分布在白海[14]、地中海[15]、含盐水体[16]、云南滇池[17]等淡水和含盐水生生态系统[14–18],也分布在湿地和旱地等陆地生态系统[3, 19–21]。湿度和pH是指示北极土壤紫色AnPB多样性变化的重要因子[22];盐度和养分是影响青藏高原不同海拔高度土壤AnPB类群空间异质性的重要因素[23];盐度、光照条件和营养梯度等是影响AnPB群落结构和遗传多样性的关键因素[24]。迄今为止,AnPB在水生、湿生和旱生复合生境下的空间分布特征及影响因素尚不明晰。
锡林河流域集水生、湿生和旱生环境于一体,是研究AnPB陆向分异及生物多样性和生态系统功能(biodiversity and ecosystem functioning,BEF)的理想场所。Imhoff研究证明16S rRNA基因是研究AnPB的生物标志[16]。本研究基于前人文献[1–26]和16S rRNA基因高通量测序技术,研究锡林河流域潜在光合固碳AnPB群落科水平组成的陆向分布特征及适宜的环境条件,以期为探究锡林河流域AnPB生物多样性与生态系统功能以及降低CO2排放和增加土壤碳汇提供科学导向,亦可为制备AnPB生物肥料及挖掘污染物降解菌提供科学依据。
1 材料与方法 1.1 研究区概况与样品的采集锡林河流域(43°39'–44°36'N、115°33'–117°28'E)位于我国温带半干旱大陆性季风气候区,近60年的年均温为2.6 ℃,年平均降水量为296.3 mm,其中5–8月份降水量占全年的51%–89%[27]。代表性水生植被为水莎草(Juncellus serotinus)、湿生植被为灯芯草(Juncus effusus)和鹅绒委陵菜(Potentilla anserina),旱生植被为羊草(Leymus chinensis)和大针茅(Stipa grandis)[27]。代表性的土壤包括长期淹水区湍流带砂质寡营养土壤和缓滞流带黑臭(推测含有硫化物和氨态氮等)粉质土壤[27]、季节性淹水区沼泽化草甸土等。
采样时间为2020年7月5日,沿着由水及陆的方向依次采集长期淹水的河床湍流带Np、缓流带Js、滞流带Pb,季节性淹水的河滨灯芯草样带Je和鹅绒委陵菜样带Pa,长期不淹水的阶地羊草样带Lc和大针茅样带Sg土壤样品(图 1)。其中,河床湍流带Np、缓流带Js、滞流带Pb代表水生环境;灯芯草样带Je代表水偏湿生环境、鹅绒委陵菜样带Pa代表湿偏旱生环境;羊草样带Lc和大针茅样带Sg均代表旱生环境。每个样带沿着河流走向依次设计3个样点(样点间距500–2 000 m),7个样带共21个样点。样带Np–Pa的中心间距10–100 m,样带Lc–Sg的中心间距100–1 000 m,样带Pa–Lc的中心间距大于1 km。本研究采集0–10 cm表层土壤,每个采样点均按照S型进行5点取样,混合后制备成1个样品(约1 kg)置于无菌塑料袋中,24 h内用冰盒运到实验室。按照四分法分取样品,部分样品–80 ℃保存供分子生物学分析,其余土样风干、研磨、过筛供理化因子检测。
|
| 图 1 锡林河流域7个采样带的陆向梯度 Figure 1 Landward gradient of seven sampling zones from the Xilin River Basin. Seven sampling zones included long-term flooding riverbed (Np, Js, Pb), seasonal flooding riverside (Je, Pa) and no-flooding terrace (Lc, Sg); Np, Js, Pb respectively represented the turbulent flow zone, sluggish flow zone, stagnant flow zone in aquatic habitat; Je and Pa respectively represented semi-aquatic and semi-xerophytic hygrophytic habitats; Lc and Sg respectively represented xerophytic Leymus chinensis and Stipa grandis. |
1.2 理化因子的检测
土壤样品的理化指标检测主要依照《土壤农化分析》[28]中的方法进行。其中,湿度(moisture)含量采用105 ℃烘干称重法测定;盐度(salinity)利用DDS-11A型电导率仪测定;总有机碳(total organic carbon,TOC)含量采用重铬酸钾外加热法测定;全氮(total nitrogen,TN)含量采用凯式定氮法测定;pH值采用电极电位法(水土比2.5:1)测定;黏粒(clay)、粉粒(silt)、砂粒(sand)含量利用激光粒度分析仪(Mastersizer 2000,Malvern Panalytical)进行测定。所有理化指标测定3次重复。
1.3 土壤潜在AnPB类群的检测及生物信息学分析按照FastDNA Spin Kit for Soil (MP Biomedicals,Southern California)说明书提取土壤样品中微生物总DNA。土壤潜在AnPB类群的16S rRNA基因PCR扩增引物为细菌V3–V4区的338F (5'-ACTCCTACGGGAGGCAGCA-3')和806R (5'-G GACTACHVGGGTWTCTAAT-3')[29]。具体扩增反应体系和工作条件见文献[29]。依托北京百迈客生物科技有限公司Illumina MiSeq PE300测序平台进行高通量测序。
使用Trimmomatic软件删除测序接头、非特异性扩增片段、模糊碱基、单碱基高重复区及长度过短的序列以获得高质量序列;使用Flash软件将双端测序的基因序列进行拼接。运用UPARSE软件在97%相似度的水平上对可操作分类单元(operational taxonomic unit,OTU)进行聚类分析,同时剔除嵌合体。使用RDP classifier对每个OTU中的序列进行分类学注释,总共获得373个细菌科。运用SILVA数据库将全部样品获取的373个细菌科在每份样品中分配的菌科及其对应的序列数进行组成的划分和相对丰度的计算。所有测序数据已上传至NCBI数据库,序列号为SRR8835404–8835405、8835408、8835410、8835412–8835413、8835415–8835417、8835419、8835421–8835425、8835427–8835429、8835432、8835434–8835435。参照前人文献[1–26]在科水平筛选出15个潜在AnPB类群进行系统发育分析。
1.4 数据的统计与分析使用Excel 2010进行基本数据的处理和表格制作;使用Origin 2021进行柱状图的绘制;使用MEGA 6.0构建系统发育树;使用SPSS 26.0进行单因素方差分析(analysis of variance,ANOVA)、Games-Howell检验以及单一理化因子与单一AnPB的Pearson相关性分析。使用CANOCO 5.0 (Biometris-Plant Research International,Wageningen)对不同样品AnPB与理化因子进行多重相关的冗余分析(redundancy analysis,RDA)。利用R语言vegan程序包绘制聚类热图;利用mvpart程序包[30]构建多元回归树(multivariate regression tree,MRT);利用lavaan程序包[31]构建结构方程模型(structural equation modeling,SEM)。
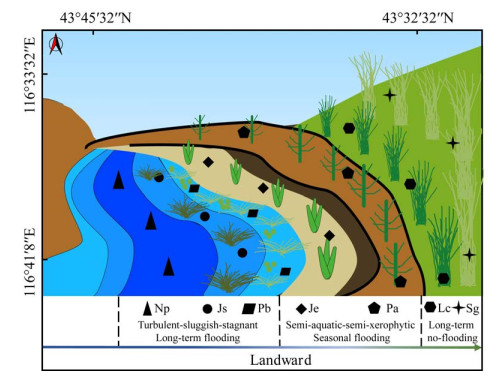
2 结果与分析 2.1 锡林河流域不同样带土壤理化因子的陆向分异规律沿着长期性淹水的水生环境、季节性淹水的湿生环境、长期性不淹水的旱生环境梯度,土壤湿度、总有机碳、黏粒、粉粒含量呈现先升高后降低的陆向分布趋势(图 2),分别在水生缓流带Js、滞流带Pb、旱生样带Lc、旱生样带Lc达到峰值;土壤全氮含量呈现先升高后降低再升高的陆向分布趋势(图 2),在旱生样带Lc达到峰值;土壤盐度和pH大致呈现逐渐升高的陆向分布趋势(图 2),分别在旱生样带Lc和水生滞流带Pb达到峰值;砂粒含量大致呈现逐渐降低的陆向分布趋势(图 2),在水生湍流带Np达到峰值。水生湍流带Np土壤总有机碳、全氮、盐度、粉粘粒含量显著(P < 0.05)低于其他样带,说明水生湍流带Np为砂质寡营养环境,盐度相对较低。水生缓流带Js及滞流带Pb和湿生环境的盐度(1.22–1.29 mg/kg)显著(P < 0.05)低于旱生环境的盐度(1.75–1.84 mg/kg);旱生环境的土壤湿度显著(P < 0.05)低于水生缓流带Js及滞流带Pb和湿生环境;除水生湍流带Np外,所有样带土壤pH值(8.19–8.75)差异不显著(P > 0.05)。
|
| 图 2 锡林河流域河床(Np、Js、Pb)-河滨(Je、Pa)-阶地(Lc、Sg) 7个样带土壤理化因子的陆向分异 Figure 2 Landward differentiation of the soil physicochemical factors from seven sampling zones in riverbed (Np, Js, Pb)-riverside (Je, Pa)-terrace (Lc, Sg) in Xilin River Basin. The same and different lowercase letters respectively indicated insignificant (P > 0.05) and significant (P < 0.05) differences of samples which were determined by one-way analysis of variance (ANOVA) followed by Games-Howell's test; all indicators were detected in triplicate. |
2.2 锡林河流域不同样带潜在AnPB的系统发育特征
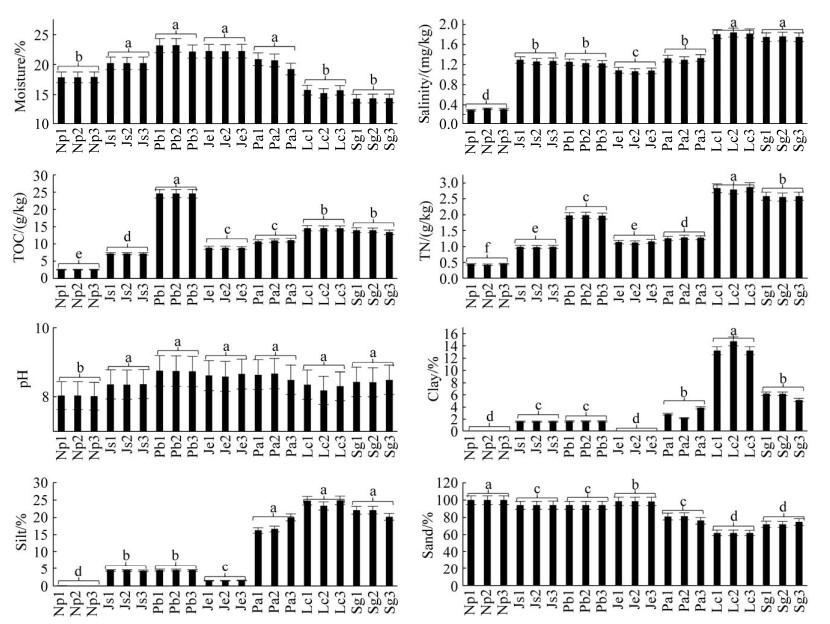
本研究检测到的AnPB主要包括来自γ-变形菌纲(Gammaproteobacteria)的着色杆菌科(Chromatiaceae)和外硫红螺菌科(Ectothiorhodospiraceae)等2个科,属于紫色硫细菌PSB;来自α-和β-变形菌纲(Alpha和Betaproteobacteria)的红杆菌科(Rhodobacteraceae)、红环菌科(Rhodocyclaceae)、红螺菌科(Rhodospirillaceae)、醋酸杆菌科(Acetobacteraceae)、慢生根瘤菌科(Bradyrhizobiaceae)、生丝微菌科(Hyphomicrobiaceae)、红菌科(Rhodobiaceae)、丛毛单胞菌科(Comamonadaceae)等8个科,属于紫色非硫细菌PNSB;来自绿菌门(Chlorobi)的绿菌科(Chlorobiaceae),属于绿色硫细菌GSB;来自绿弯菌门(Chloroflexi)的蔷薇菌科(Roseiflexaceae),属于绿色非硫细菌GNSB;来自酸杆菌门(Acidobacteria)的酸杆菌科(Acidobacteriaceae)和全噬菌科(Holophagaceae);来自芽单胞菌门(Gemmatimonadetes)的芽单胞菌科(Gemmatimonadaceae) (图 3)。
|
| 图 3 锡林河流域潜在AnPB 16S rRNA基因系统发育树 Figure 3 Phylogenetic tree of potential anoxygenic photosynthetic bacteria (AnPB) based on 16S rRNA gene sequences in Xilin River Basin. The up-triangle and down-triangle respectively represented the AnPB and out-group bacteria detected in this study. All detected and reference AnPB as well as out-group bacteria were uncultured; dark purple, light purple, orange, dark green, yellow and light green represented purple non-sulfur bacteria (PNSB), purple sulfur bacteria (PSB), green non-sulfur bacteria (GNSB), green sulfur bacteria (GSB), Acidobacter and Gemmatimonadete, respectively; while blue color represented out-group bacteria. |
2.3 锡林河流域不同样带潜在AnPB的陆向分异规律
沿着长期性淹水的水生环境、季节性淹水的湿生环境、长期性不淹水的旱生环境梯度,潜在AnPB类群中PNSB丛毛单胞菌科(Comamonadaceae)的相对丰度呈现逐渐降低的陆向分布趋势(图 4),最高相对丰度出现在水生滞流带Pb (Comamonadaceae,7.72%);PSB中着色杆菌科(Chromatiaceae)、PNSB中红环菌科(Rhodocyclaceae)、全噬菌科(Holophagaceae)、GSB中绿菌科(Chlorobiaceae)、PNSB中红杆菌科(Rhodobacteraceae)、PNSB中醋酸杆菌科(Acetobacteraceae)、PNSB中生丝微菌科(Hyphomicrobiaceae)、GNSB中蔷薇菌科(Roseiflexaceae)、PSB中外硫红螺菌科(Ectothiorhodospiraceae)、酸杆菌科(Acidobacteriaceae)的相对丰度呈现先升高后降低的陆向分布趋势(图 4),最高相对丰度依次出现在水生缓流带Js (Chromatiaceae,0.19%)、Js (Rhodocyclaceae,7.31%)、Js (Holophagaceae,1.18%)、Js (Chlorobiaceae,0.32%)、水生滞流带Pb (Rhodobacteraceae,2.98%)、Pb (Acetobacteraceae,1.29%)、水偏旱生样带Pa (Hyphomicrobiaceae,2.24%)、Pa (Roseiflexaceae,0.03%)、Pa (Ectothiorhodospiraceae,0.04%)、旱生样带Lc (Acidobacteriaceae,0.14%);PNSB中红螺菌科(Rhodospirillaceae)、PNSB中慢生根瘤菌科(Bradyrhizobiaceae)和PNSB中红菌科(Rhodobiaceae)的相对丰度呈现逐渐升高的陆向分布趋势(图 4),最高相对丰度依次出现在水偏旱生的样带Pa (Rhodospirillaceae,1.80%)、旱生的样带Sg (Bradyrhizobiaceae,2.74%)和样带Lc (Rhodobiaceae,0.17%);芽单胞菌科(Gemmatimonadaceae)的相对丰度呈现先升高再降低后升高的陆向分布趋势(图 4),最高相对丰度出现在旱生样带Lc (Gemmatimonadaceae,3.86%)。
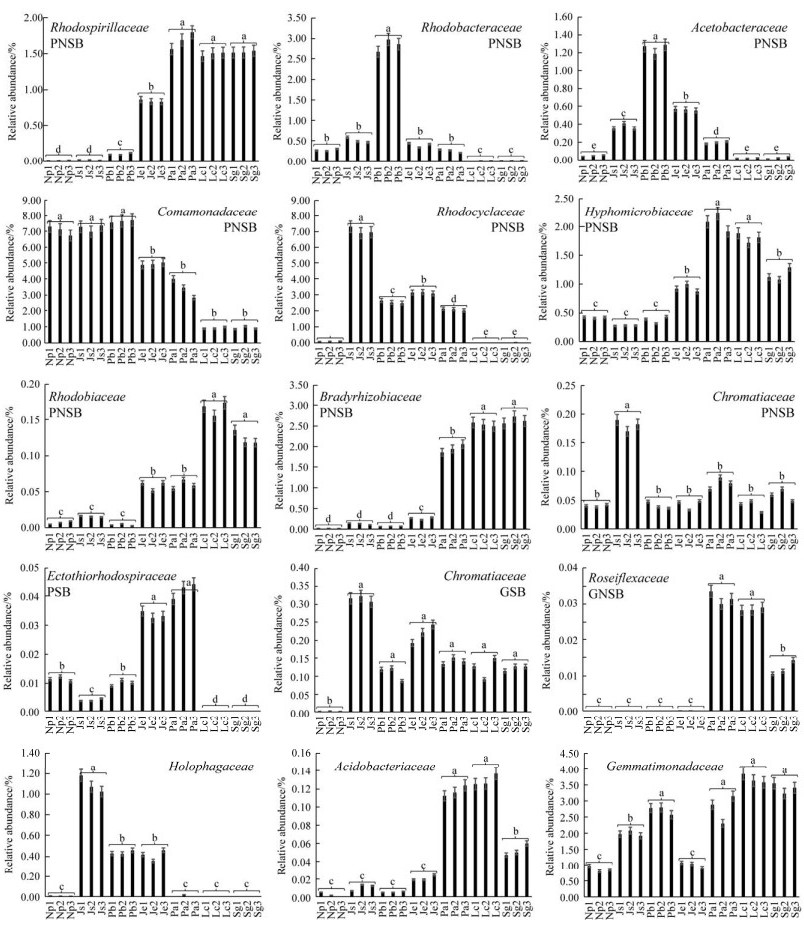
|
| 图 4 锡林河流域河床(Np、Js、Pb)-河滨(Je、Pa)-阶地(Lc、Sg) 7个样带土壤潜在AnPB的陆向分异 Figure 4 Landward differentiation of potential anoxygenic photosynthetic bacteria (AnPB) from seven sampling zones in riverbed (Np, Js, Pb)-riverside (Je, Pa)-terrace (Lc, Sg) in Xilin River Basin. The same and different lowercase letters respectively indicated insignificant (P > 0.05) and significant (P < 0.05) differences of samples which were determined by one-way analysis of variance (ANOVA) followed by Games-Howell's test. |
2.4 锡林河流域不同样带潜在AnPB不同类群的相似性和差异性
15个潜在AnPB类群相对丰度的聚类热图(图 5)表明,PNSB中红环菌科(Rhodocyclaceae)与红杆菌科(Rhodobacteraceae)的相似度较高聚为一小簇,芽单胞菌科(Gemmatimonadaceae)与PNSB中生丝微菌科(Hyphomicrobiaceae)及丛毛单胞菌科(Comamonadaceae)的相似度较高聚为一小簇,PNSB中醋酸杆菌科(Acetobacteraceae)与PSB中着色杆菌科(Chromatiaceae)及PNSB中的慢生根瘤菌科(Bradyrhizobiaceae)与红螺菌科(Rhodospirillaceae)的相似度较高分别聚为一小簇,酸杆菌科(Acidobacteriaceae)与GSB中绿菌科(Chlorobiaceae)及全噬菌科(Holophagaceae)与PSB中外硫红螺菌科(Ectothiorhodospiraceae)的相似度较高分别聚为一小簇。
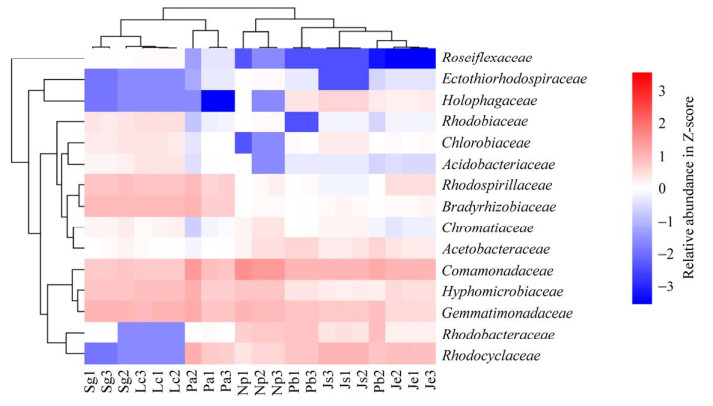
|
| 图 5 锡林河流域河床(Np、Js、Pb)-河滨(Je、Pa)-阶地(Lc、Sg) 7个样带潜在AnPB不同类群的聚类热图分析 Figure 5 Heat-map based clustering analysis of different groups of potential anoxygenic photosynthetic bacteria (AnPB) from seven sampling zones in riverbed (Np, Js, Pb)-riverside (Je, Pa)-terrace (Lc, Sg) in Xilin River Basin. |
由15个潜在AnPB类群相对丰度的相似度分析(图 5)可知,水生湍流带Np、缓流带Js、滞流带Pb、水偏湿生样带Je聚为一大类;水偏旱生样带Pa、旱生样带Sg和Lc聚为一大类。
2.5 锡林河流域不同样带潜在AnPB类群与理化因子的相关性Pearson相关性分析结果(表 1)显示,PNSB中红螺菌科(Rhodospirillaceae)、慢生根瘤菌科(Bradyrhizobiaceae)、生丝微菌科(Hyphomicrobiaceae)和红菌科(Rhodobiaceae)、GNSB中蔷薇菌科(Roseiflexaceae)、酸杆菌门中酸杆菌科(Acidobacteriaceae)和芽单胞菌门中芽单胞菌科(Gemmatimonadaceae)的相对丰度与盐度(生丝微菌科除外)和全氮含量(生丝微菌科、蔷薇菌科除外)呈极显著(P < 0.01)正相关关系;PNSB中红杆菌科(Rhodobacteraceae)、红环菌科(Rhodocyclaceae)、醋酸杆菌科(Acetobacteraceae)和丛毛单胞菌科(Comamonadaceae)的相对丰度与湿度呈极显著(P < 0.01)正相关关系;PSB中外硫红螺菌科(Ectothiorhodospiraceae)和酸杆菌门中全噬菌科(Holophagaceae)的相对丰度与湿度呈显著(P < 0.05)正相关关系;PNSB中生丝微菌科(Hyphomicrobiaceae)的相对丰度与盐度和全氮含量均呈显著(P < 0.05)正相关关系。
| AnPB families | Moisture | Salinity | TOC | TN | pH | Clay | Silt | Sand |
| Chromatiaceae | 0.138 | 0.094 | –0.281 | –0.295 | –0.046 | –0.192 | –0.130 | 0.155 |
| Ectothiorhodospiraceae | 0.544* | –0.261 | –0.128 | –0.412 | 0.457* | –0.418 | –0.156 | 0.249 |
| Rhodobacteraceae | 0.643** | –0.121 | 0.720** | 0.029 | 0.552** | –0.324 | –0.407 | 0.394 |
| Rhodocyclaceae | 0.614** | –0.060 | –0.108 | –0.423 | 0.286 | –0.450* | –0.474* | 0.482* |
| Rhodospirillaceae | –0.490* | 0.673** | 0.137 | 0.592** | 0.189 | 0.613** | 0.867** | –0.812** |
| Acetobacteraceae | 0.796** | –0.095 | 0.654** | –0.041 | 0.696** | –0.418 | –0.494* | 0.486* |
| Bradyrhizobiaceae | –0.736** | 0.763** | 0.195 | 0.748** | –0.013 | 0.789** | 0.980** | –0.949** |
| Hyphomicrobiaceae | –0.350 | 0.536* | 0.084 | 0.454* | 0.151 | 0.599** | 0.796** | –0.757** |
| Rhodobiaceae | –0.737** | 0.759** | 0.137 | 0.789** | –0.109 | 0.881** | 0.876** | –0.908** |
| Comamonadaceae | 0.735** | –0.731** | –0.109 | –0.729** | 0.040 | –0.776** | –0.912** | 0.898** |
| Chlorobiaceae | 0.324 | 0.358 | –0.049 | –0.047 | 0.359 | –0.100 | –0.079 | 0.089 |
| Roseiflexaceae | –0.416 | 0.560** | 0.129 | 0.493* | 0.005 | 0.728** | 0.846** | –0.835** |
| Acidobacteriaceae | –0.438* | 0.626** | 0.160 | 0.565** | 0.031 | 0.778** | 0.870** | –0.869** |
| Holophagaceae | 0.526* | –0.051 | –0.039 | –0.326 | 0.196 | –0.390 | –0.518* | 0.493* |
| Gemmatimonadaceae | –0.493* | 0.873** | 0.625** | 0.884** | 0.207 | 0.795** | 0.887** | –0.886** |
| *: correlation was significant at the 0.05 level (2-tailed); **: correlation was significant at the 0.01 level (2-tailed). | ||||||||
RDA结果(图 6)显示,第一排序轴与第二排序轴的解释度分别为71.49%和14.15%,PNSB中丛毛单胞菌科(Comamonadaceae)、红杆菌科(Rhodobacteraceae)、红环菌科(Rhodocyclaceae)和醋酸杆菌科(Acetobacteraceae)、酸杆菌门中全噬菌科(Holophagaceae)、PSB中外硫红螺菌科(Ectothiorhodospiraceae)和着色杆菌科(Chromatiaceae)与砂粒、湿度和pH关联度较高;芽单胞菌科(Gemmatimonadaceae)、PNSB中红菌科(Rhodobiaceae)、生丝微菌科(Hyphomicrobiaceae)、慢生根瘤菌科(Bradyrhizobiaceae)和红螺菌科(Rhodospirillaceae)、GNSB中蔷薇菌科(Roseiflexaceae)、酸杆菌门中酸杆菌科(Acidobacteriaceae)和GSB中绿菌科(Chlorobiaceae)与粘粒、全氮、粉粒和盐度关联度较高。
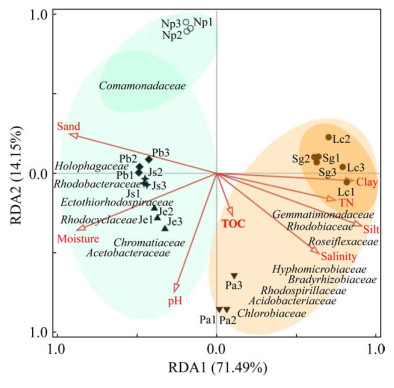
|
| 图 6 锡林河流域河床(Np、Js、Pb)-河滨(Je、Pa)-阶地(Lc、Sg) 7个样带土壤理化因子与潜在AnPB的冗余分析 Figure 6 Redundancy analysis (RDA) between the soil physicochemical factors and potential anoxygenic photosynthetic bacteria (AnPB) from seven sampling zones in riverbed (Np, Js, Pb)-riverside (Je, Pa)-terrace (Lc, Sg) in Xilin River Basin. |
一级和二级分支盐度对潜在AnPB陆向分异的总解释度分别为57.56%和4.83%,全氮的总解释度为12.68%,一级和二级分支湿度的总解释度分别为7.74%和6.27% (图 7和表 2)。二级分支盐度对PNSB中丛毛单胞菌科(Comamonadaceae)陆向分异的解释度为32.82%和2.56%,二级分支湿度对PNSB中丛毛单胞菌科(Comamonadaceae)陆向分异的解释度为1.46%和1.81%,全氮对PNSB中丛毛单胞菌科(Comamonadaceae)陆向分异的解释度为0.06%;二级分支盐度对PNSB中红环菌科(Rhodocyclaceae)陆向分异的解释度为9.35%和1.83%,全氮对PNSB中红环菌科(Rhodocyclaceae)陆向分异的解释度为11.49%,二级分支湿度对PNSB中红环菌科(Rhodocyclaceae)陆向分异的解释度为0.49%和3.84%;二级分支盐度对PNSB中慢生根瘤菌科(Bradyrhizobiaceae)陆向分异的解释度为6.47%和0.16%,全氮对PNSB中慢生根瘤菌科(Bradyrhizobiaceae)陆向分异的解释度为0.17%,二级分支湿度对PNSB中慢生根瘤菌科(Bradyrhizobiaceae)陆向分异的解释度为0.002%和0.03%;二级分支盐度对芽单胞菌科(Gemmatimonadaceae)陆向分异的解释度为3.98%和0.11%,全氮对芽单胞菌科(Gemmatimonadaceae)陆向分异的解释度为0.22%,二级分支湿度对芽单胞菌科(Gemmatimonadaceae)陆向分异的解释度为1.72%和0.008%;二级分支盐度对PNSB中红螺菌科(Rhodospirillaceae)陆向分异的解释度为1.95%和0.01%,全氮对PNSB中红螺菌科(Rhodospirillaceae)陆向分异的解释度为0.01%,二级分支湿度对PNSB中红螺菌科(Rhodospirillaceae)陆向分异的解释度为0.06%和0.28%;二级分支盐度对PNSB中红杆菌科(Rhodobacteraceae)陆向分异的解释度为1.07%和0.02%,全氮对PNSB中红杆菌科(Rhodobacteraceae)陆向分异的解释度为0.39%,二级分支湿度对PNSB中红杆菌科(Rhodobacteraceae)陆向分异的解释度为3.45%和0.006%。盐度、全氮和湿度对其他潜在AnPB类群的解释度较低。
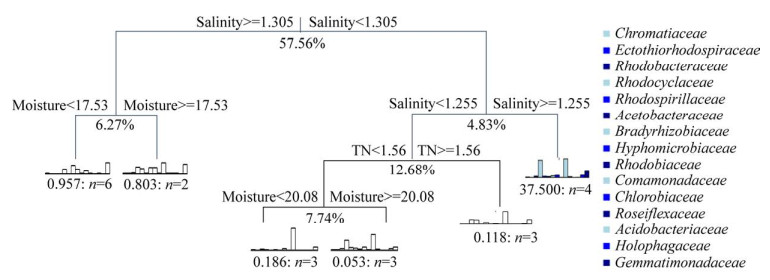
|
| 图 7 锡林河流域河床(Np、Js、Pb)-河滨(Je、Pa)-阶地(Lc、Sg) 7个样带土壤理化因子与潜在AnPB类群的多元回归树分析 Figure 7 Multivariate regression tree (MRT) analysis between the soil physicochemical factors and potential anoxygenic photosynthetic bacteria (AnPB) populations from seven sampling zones in riverbed (Np, Js, Pb)-riverside (Je, Pa)-terrace (Lc, Sg) in Xilin River Basin. |
| Species | Species variance explained by tree splits and whole tree/% | ||||||
| Salinity < 1.305 | Salinity < 1.255 | TN < 1.56 | Moisture < 20.08 | Moisture < 17.53 | Tree total | Species total | |
| Chromatiaceae | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 |
| Ectothiorhodospiraceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Rhodobacteraceae | 1.07 | 0.02 | 0.39 | 3.45 | 0.01 | 4.94 | 4.97 |
| Rhodocyclaceae | 9.35 | 1.83 | 11.49 | 0.49 | 3.84 | 26.99 | 32.05 |
| Rhodospirillaceae | 1.95 | 0.01 | 0.01 | 0.06 | 0.28 | 2.31 | 2.90 |
| Acetobacteraceae | 0.29 | 0.01 | 0.06 | 0.49 | 0.11 | 0.97 | 0.98 |
| Bradyrhizobiaceae | 6.47 | 0.16 | 0.17 | 0.00 | 0.03 | 6.83 | 7.53 |
| Hyphomicrobiaceae | 1.32 | 0.11 | 0.03 | 0.05 | 0.11 | 1.61 | 2.59 |
| Rhodobiaceae | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 |
| Comamonadaceae | 32.82 | 2.56 | 0.06 | 1.46 | 1.81 | 38.71 | 41.88 |
| Chlorobiaceae | 0.00 | 0.00 | 0.02 | 0.00 | 0.02 | 0.04 | 0.05 |
| Roseiflexaceae | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Acidobacteriaceae | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| Holophagaceae | 0.28 | 0.00 | 0.22 | 0.03 | 0.07 | 0.60 | 0.84 |
| Gemmatimonadaceae | 3.98 | 0.11 | 0.22 | 1.72 | 0.01 | 6.05 | 6.16 |
| Total interpretation | 57.56 | 4.83 | 12.68 | 7.74 | 6.27 | 89.09 | 100.00 |
SEM拟合结果显示,盐度对PNSB中红环菌科(Rhodocyclaceae)、红螺菌科(Rhodospirillaceae)和慢生根瘤菌科(Bradyrhizobiaceae)及芽单胞菌科(Gemmatimonadaceae)的陆向分异发挥正向直接作用,路径系数分别为1.28、0.76、0.60和0.52,对PNSB中红杆菌科(Rhodobacteraceae)和丛毛单胞菌科(Comamonadaceae)的陆向分异发挥负向直接作用,路径系数分别为–0.89和–0.53;全氮对PNSB中红杆菌科(Rhodobacteraceae)、芽单胞菌科(Gemmatimonadaceae)和PNSB中丛毛单胞菌科(Comamonadaceae)的陆向分异发挥正向直接作用,路径系数分别为1.30、0.44和0.01,对PNSB中红环菌科(Rhodocyclaceae)、红螺菌科(Rhodospirillaceae)和慢生根瘤菌科(Bradyrhizobiaceae)的陆向分异发挥负向直接作用,路径系数分别为–1.33、–0.23和–0.05;湿度对PNSB中红杆菌科(Rhodobacteraceae)、红环菌科(Rhodocyclaceae)和丛毛单胞菌科(Comamonadaceae)的陆向分异发挥正向直接作用,路径系数分别为0.95、0.44和0.44,对PNSB中慢生根瘤菌科(Bradyrhizobiaceae)和红螺菌科(Rhodospirillaceae)及芽单胞菌科(Gemmatimonadaceae)的陆向分异发挥负向直接作用,路径系数为–0.53、–0.32和–0.01。盐度、全氮和湿度还可通过彼此间相互作用对潜在AnPB陆向分异发挥间接作用(图 8)。
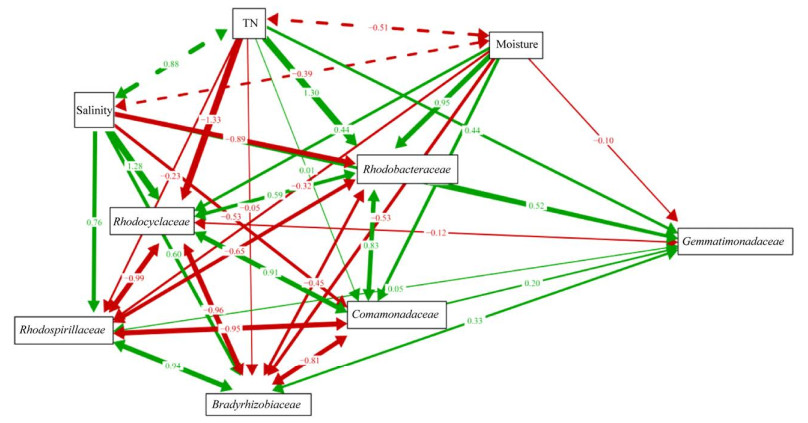
|
| 图 8 锡林河流域河床(Np、Js、Pb)-河滨(Je、Pa)-阶地(Lc、Sg) 7个样带土壤理化因子与优势AnPB的结构方程模型 Figure 8 Structural equation modeling (SEM) between the soil physicochemical factors and dominant anoxygenic photosynthetic bacteria (AnPB) from seven sampling zones in riverbed (Np, Js, Pb)-riverside (Je, Pa)-terrace (Lc, Sg) in Xilin River Basin. Dashed and solid arrows represented insignificant and significant relationships, respectively; the size of the pathway coefficients was defined as important values, and green and red arrows indicated positive and negative relationships, respectively. |
3 讨论 3.1 锡林河流域不同样带潜在AnPB类群陆向分异特征
本研究发现相比于PSB、GSB、GNSB、酸杆菌门及芽单胞菌门7个AnPB类群,8个PNSB类群是锡林河流域组成丰度最丰富、分布最广泛的潜在AnPB类群,这可能与PNSB在进化过程中具有高度变异性有关。PNSB营养及代谢类型多样化,参与卡尔文循环、3-HP循环、DC/HB和HP/HB循环耦联异化硝酸盐还原、固氮、反硝化及还原态硫氧化和异化硫酸盐还原(表 3)[32],说明PNSB是碳、氮、硫循环的主要驱动者,这为本研究PNSB适应于不同基质营养环境的结果提供了间接支撑。本研究发现PNSB能适应各种水分和盐度环境,这与文献报道的PNSB能适应广泛的水分和盐度环境且具有生境高度异质性的研究结果[9]相一致,推测PNSB与各种环境生物存在基因的水平/侧向传递(horizontal/lateral gene transfer,HGT/LGT)作用[9]。本研究中PSB中着色杆菌科和GSB中绿菌科在长期淹水缓流带Js黑臭粉质土壤中的相对丰度最高,推测该样带土壤硫化物含量[33]适宜于PSB和GSB生长繁殖,氮磷含量相对过剩导致PSB和GSB水体富营养化现象。研究表明水生环境中GSB和PSB存在分层分布现象,PSB对光的依赖性强分布在相对强光缺氧的上层,不能进行有氧呼吸[32];而GSB对光的依赖性不强适应于严格厌氧的下层[9],推测锡林河流域长期淹水的缓流带Js中PSB和GSB存在生态位分化现象。GSB中Prosthecochloris aestaurii既可与异养菌Geobacter sulfurreducens发生种间电子传递作用间接固定CO2,也可在厌氧光照条件下直接固定CO2[34],说明GSB具有固碳途径的多样性,这为本研究GSB可能具有多种固碳途径提供间接科学依据。锡林河流域PNSB、PSB、GNSB和GSB能否参与卡尔文循环、Wood-Ljungdahl循环(GNSB)、3-HP循环、DC/HB和HP/HB循环、rTCA、发酵、异化硝酸盐还原、固氮、反硝化及还原态硫氧化和异化硫酸盐还原等碳、氮、硫循环,有待于后续研究证实。
| Cycle | Metabolic pathway | PNSB | PSB | GNSB | GSB |
| Carbon cycle | Calvin cycle | + | + | + | + |
| Wood-Ljungdahl | + | ||||
| 3-HP bicycle | + | + | + | ||
| DC/HB and HP/HB cycles | + | + | + | + | |
| rTCA (ACL) | + | ||||
| Fermentation | + | + | + | + | |
| Nitrogen cycle | Dissimilatory nitrate reduction | + | + | + | + |
| Nitrogen fixation | + | + | + | + | |
| Denitrification | + | + | + | + | |
| O2/NO respiration | + | + | + | ||
| Sulfur cycle | Dissimilatory sulfate reduction | + | + | + | + |
| Sulfur oxidation system | + | + | + | + | |
| PNSB from Alphaproteobacteria and Betaproteobacteria; PSB from Gammaproteobacteria; GNSB from Chloroflexi; GSB from Chlorobi; 3-HP: 3-hydroxyproprionate; DC/HB: dicarboxylate/hydrobutyrate; HP/HB: 3-hydroxyproprionate/ 4-hydrobutyrate; rTCA: reverse tricarboxylic acid cycle. +: presence of the metabolic pathway. | |||||
PNSB中丛毛单胞菌科沿陆向呈现逐渐降低的分布趋势,是湍流带裸砂相对寡营养(图 2)水生环境较优势的潜在AnPB,这与丛毛单胞菌科分布在白海沿岸矿质营养匮乏环境的研究结果[14]具有相似性,说明丛毛单胞菌科可适应于寡营养的水生环境。锡林河流域丛毛单胞菌科也分布于缓流带、滞流带和水偏湿生样带土壤中,这与丛毛单胞菌科适应于云南滇池[17]、丛毛单胞菌科主要分布于水生和湿生环境[19]的研究结果相一致。锡林河流域PNSB中红环菌科沿陆向呈现先升高后降低的分布趋势,最适于水生缓流带土壤环境、较适于滞流带和湿生土壤环境。华盛顿湖泊沉积物中甲基营养型红环菌科适应于淹水环境[18]、红杆菌科为夏季地中海的优势类群[15],这些文献为本研究PNSB中红环菌科和红杆菌科适于水生和湿生环境的结果提供了科学依据。Imhoff[16]利用16S rRNA基因测序和系统发育分析证明PSB中外硫红螺菌科和着色杆菌科适应于含盐及海洋环境,这为本研究基于16S rRNA基因高通量测序证明锡林河流域着色杆菌科最适于水生缓流带(图 2)的结果提供了支撑。芽单胞菌科对干旱土壤环境具有耐受性[11]、慢生根瘤菌科适应于金沙江干热河谷区土壤环境[20]、红螺菌科适于沙漠和岩石生物膜等干旱环境[21],这些文献为本研究芽单胞菌科、PNSB中慢生根瘤菌科和红螺菌科主要分布在旱生土壤环境的结果提供了科学支撑。
3.2 锡林河流域不同样带潜在AnPB类群陆向分异的环境影响因素盐度与锡林河流域湿偏旱生和旱生环境中优势的PNSB中4个类群,芽单胞菌科、酸杆菌科和GNSB等呈显著(P < 0.05)或极显著(P < 0.01)正相关关系,这与PNSB中红环菌科对高盐环境具有耐受性[35]、盐胁迫提高芽单胞菌科相对丰度[36]、PNSB中慢生根瘤菌科是盐矿中的优势菌[37]、嗜盐PNSB中红螺菌科生存在盐度1%–10%的沿海环境[38–39]等研究结果具有一致性,说明锡林河流域湿偏旱生和旱生环境中的优势AnPB为耐盐菌或嗜盐菌。盐度与锡林河流域水生及水偏湿生环境中优势PSB、PNSB中其他4个类群、全噬菌科呈负相关关系,这与PNSB中红杆菌科存在高亲和力的运转体来适应低盐环境[40]的研究结果具有相似性,说明锡林河流域水生及水偏湿生环境中优势AnPB更适应于低盐环境。综上所述,锡林河流域潜在AnPB类群既能适应于相对低盐环境也能适应于相对高盐环境,对盐度的适应范围较广(8%–11% NaCl)[9]。这与Imhoff[16]证明AnPB类群适应于海洋、盐湖和淡水等高盐和低盐环境,是盐度变化的模式生物的研究结果具有相似性。原因可能是PNSB通过能量来维持胞内的渗透压以适应更广泛的盐度范围(0–25 g/L NaCl);嗜盐菌PSB和GSB通过Na+/H+交换泵中的逆向转运蛋白偶联H+输入将胞内过剩的Na+泵出从而维持胞内外渗透压以适应盐基饱和环境[41];PSB中外硫红螺菌和PNSB中的红杆菌及慢生根瘤菌能够合成甜菜碱(glycine betaine)作为渗透保护剂(osmoprotectants)来抵御高盐环境[42–44]。
湿度与锡林河流域水生及水偏湿生环境优势PNSB中红环菌科、红杆菌科和丛毛单胞菌科呈现极显著(P < 0.01)正相关关系,这与Imhoff[16]研究发现AnPB广泛分布于海洋、盐湖和淡水等水生环境的结果相一致;与PNSB中红杆菌科是地中海东部水域生物膜形成过程的关键细菌[15]、PNSB中红环菌科分布于地下浅层含水层[45]和太湖沉积物[46]、PNSB中丛毛单胞菌科分布在淡水湖泊水生环境[47]的研究结果相似,说明土壤湿度是影响锡林河流域潜在AnPB类群陆向分异的重要环境因子。
全氮是仅次于盐度和湿度的第三大环境因子,这与PNSB中红环菌科是反硝化脱氮过程的优势菌[48]、红环菌科是活性污泥中重要的硝酸盐还原菌[49]、芽单胞菌科是活性污泥脱氮功能的调节者[50]对氮供应做出正向响应[51]、慢生根瘤菌科等AnPB具有固氮活性[4, 52–53]等研究结果相一致,说明全氮能影响锡林河流域潜在AnPB类群陆向分异特征。
4 结论锡林河流域检测到的15个潜在不产氧光合细菌科隶属于变形菌门、绿菌门、绿弯菌门、酸杆菌门和芽单胞菌门。
PSB (外硫红螺菌科和着色杆菌科)、部分PNSB (红杆菌科、红环菌科、醋酸杆菌科、丛毛单胞菌科)、全噬菌科、GSB (绿菌科)主要分布在水生及水偏湿生土壤环境;部分PNSB (红螺菌科、慢生根瘤菌科、生丝微菌科、红菌科)、芽单胞菌科、酸杆菌科、GNSB (蔷薇菌科)等主要分布在湿偏旱生和旱生土壤环境。沿着长期性淹水的水生环境、季节性淹水的湿生环境、长期性不淹水的旱生环境梯度,PNSB中丛毛单胞菌科呈现逐渐降低的陆向分布趋势;PNSB中红环菌科呈现先升高后降低的陆向分布趋势,最适于水生缓流带土壤;芽单胞菌科、PNSB中慢生根瘤菌科和红螺菌科大致呈现逐渐升高的陆向分布趋势,主要分布在旱生土壤环境。
锡林河流域潜在不产氧光合细菌既能适应于相对低盐寡氮的水生湍流环境也能适应于相对高盐富氮的水生、湿生、旱生环境。盐度、湿度和全氮分别是影响不产氧光合细菌陆向分异的第一、第二和第三大环境因子。
| [1] | George DM, Vincent AS, Mackey HR. An overview of anoxygenic phototrophic bacteria and their applications in environmental biotechnology for sustainable resource recovery. Biotechnology Reports: Amsterdam, Netherlands, 2020, 28: e00563. DOI:10.1016/j.btre.2020.e00563 |
| [2] | Thompson KJ, Simister RL, Hahn AS, Hallam SJ, Crowe SA. Nutrient acquisition and the metabolic potential of photoferrotrophic Chlorobi. Frontiers in Microbiology, 2017, 8: 1212. DOI:10.3389/fmicb.2017.01212 |
| [3] | Atamna-Ismaeel N, Finkel O, Glaser F, Von Mering C, Vorholt JA, Koblížek M, Belkin S, Béjà O. Bacterial anoxygenic photosynthesis on plant leaf surfaces. Environmental Microbiology Reports, 2012, 4(2): 209-216. DOI:10.1111/j.1758-2229.2011.00323.x |
| [4] | De Meyer SE, Coorevits A, Willems A. Tardiphaga robiniae gen. nov., sp. nov., a new genus in the family Bradyrhizobiaceae isolated from Robinia pseudoacacia in Flanders (Belgium). Systematic and Applied Microbiology, 2012, 35(4): 205-214. DOI:10.1016/j.syapm.2012.02.002 |
| [5] | Li SY, Zhao L, Han J, Liu S, Dai J, Fu G, Qiu D. Azoarcus halotolerans sp. nov., a novel member of Rhodocyclaceae isolated from activated sludge collected in Hong Kong. International Journal of Systematic and Evolutionary Microbiology, 2020, 70(11): 5799-5805. DOI:10.1099/ijsem.0.004476 |
| [6] | Mikes MC, Martin TK, Moe WM. Azospira inquinata sp. nov., a nitrate-reducing bacterium of the family Rhodocyclaceae isolated from contaminated groundwater. International Journal of Systematic and Evolutionary Microbiology, 2021, 71(12): 005172. |
| [7] | Corteselli EM, Aitken MD, Singleton DR. Erratum: Rugosibacter aromaticivorans gen. nov., sp. nov., a bacterium within the family Rhodocyclaceae, isolated from contaminated soil, capable of degrading aromatic compounds. International Journal of Systematic and Evolutionary Microbiology, 2017, 67(11): 4879. |
| [8] | Ge H, Batstone DJ, Keller J. Biological phosphorus removal from abattoir wastewater at very short sludge ages mediated by novel PAO clade Comamonadaceae. Water Research, 2015, 69: 173-182. DOI:10.1016/j.watres.2014.11.026 |
| [9] | Madigan MT, Jung DO. The purple phototrophic bacteria. First Edition. Berlin: Springer Link, 2009: 1-15. |
| [10] | Imhoff JF. Taxonomy and physiology of phototrophic purple bacteria and green sulfur bacteria. Anoxygenic Photosynthetic Bacteria, 2004, 2: 1-15. |
| [11] | Medina-Delarosa G, García-Oliva F, Alpuche-Solís ÁG, Ovando-Vázquez C, López-Lozano NE. The nutrient- improvement bacteria selected by Agave lechuguilla T. and their role in the rhizosphere community. FEMS Microbiology Ecology, 2021, 97(11): fiab137. |
| [12] | Csotonyi JT, Swiderski J, Stackebrandt E, Yurkov V. A new environment for aerobic anoxygenic phototrophic bacteria: biological soil crusts. Environmental Microbiology Reports, 2010, 2(5): 651-656. DOI:10.1111/j.1758-2229.2010.00151.x |
| [13] | Iniesto M, Moreira D, Reboul G, Deschamps P, Benzerara K, Bertolino P, Saghaï A, Tavera R, López-García P. Core microbial communities of lacustrine microbialites sampled along an alkalinity gradient. Environmental Microbiology, 2021, 23(1): 51-68. DOI:10.1111/1462-2920.15252 |
| [14] | Kublanovskaya A, Solovchenko A, Fedorenko T, Chekanov K, Lobakova E. Natural communities of carotenogenic chlorophyte Haematococcus lacustris and bacteria from the white sea coastal rock ponds. Microbial Ecology, 2020, 79(4): 785-800. DOI:10.1007/s00248-019-01437-0 |
| [15] | Elifantz H, Horn G, Ayon M, Cohen Y, Minz D. Rhodobacteraceae are the key members of the microbial community of the initial biofilm formed in Eastern Mediterranean coastal seawater. FEMS Microbiology Ecology, 2013, 85(2): 348-357. DOI:10.1111/1574-6941.12122 |
| [16] | Imhoff JF. True marine and halophilic anoxygenic phototrophic bacteria. Archives of Microbiology, 2001, 176(4): 243-254. DOI:10.1007/s002030100326 |
| [17] | Shi Y, Sun Y, Jiang Z, Ruan Z, Su J, Yu L, Zhang Y. Simplicispira lacusdiani sp. nov., a novel Betaproteobacterium isolated from a freshwater reservoir. International Journal of Systematic and Evolutionary Microbiology, 2019, 69(1): 129-133. DOI:10.1099/ijsem.0.003112 |
| [18] | Smalley NE, Taipale S, Marco PD, Doronina NV, Kyrpides N, Shapiro N, Woyke T, Kalyuzhnaya MG. Functional and genomic diversity of methylotrophic Rhodocyclaceae: description of Methyloversatilis discipulorum sp. nov. International Journal of Systematic and Evolutionary Microbiology, 2015, 65(7): 2227-2233. |
| [19] | Khan SA, Jeong SE, Park HY, Lee SS, Jeon CO. Geomonas soli gen. nov., sp. nov., a new member of the family Comamonadaceae, isolated from soil. Current Microbiology, 2020, 77(2): 286-293. DOI:10.1007/s00284-019-01814-5 |
| [20] |
Huang MY, Zhang XP, Li DY, Liang RY, Lindstrom K. Studies on drought tolerance of bradyrhizobial strains (Arachis) isolated from Jinshajiang River dry-hot valley. Chinese Journal of Applied and Environmental Biology, 2000, 6(3): 263-266.
(in Chinese) 黄明勇, 张小平, 李登煜, 梁如玉, Lindstrom K. 金沙江干热河谷区土著花生根瘤菌耐旱性初步研究. 应用与环境生物学报, 2000, 6(3): 263-266. |
| [21] | Noviana Z, Vieira S, Pascual J, Fobofou SAT, Rohde M, Spröer C, Bunk B, Overmann J. Hypericibacter terrae gen. nov., sp. nov. and Hypericibacter adhaerens sp. nov., two new members of the family Rhodospirillaceae isolated from the rhizosphere of Hypericum perforatum. International Journal of Systematic and Evolutionary Microbiology, 2020, 70(3): 1850–1860. |
| [22] | Feng Y, Grogan P, Caporaso JG, Zhang H, Lin X, Knight R, Chu H. pH is a good predictor of the distribution of anoxygenic purple phototrophic bacteria in Arctic soils. Soil Biology and Biochemistry, 2014, 74: 193-200. DOI:10.1016/j.soilbio.2014.03.014 |
| [23] | Yang HJ, Hu CX. Soil chemistry and nutrients influence the distribution of aerobic anoxygenic phototrophic bacteria and eukaryotic phototrophic microorganisms of physical soil crusts at different elevations on the Tibetan Plateau. Microbial Ecology, 2022, 83(1): 100-113. DOI:10.1007/s00248-021-01734-7 |
| [24] | Zheng Q, Liu YT, Steindler L, Jiao NZ. Pyrosequencing analysis of aerobic anoxygenic phototrophic bacterial community structure in the oligotrophic western Pacific Ocean. FEMS Microbiology Letters, 2015, 362(8): fnv034. |
| [25] | 布坎南(R. E. Buchanan)等编. 中国科学院微生物研究所《伯杰细菌鉴定手册》翻译组译. 伯杰细菌鉴定手册. 8版. 北京: 科学出版社, 1984. |
| [26] |
Yang SP, Lin ZH, Cui XH, Lian JK, Zhao CG, Qu YB. Current taxonomy of anoxygenic phototrophic bacteria—a review. Acta Microbiologica Sinica, 2008, 48(11): 1562-1566.
(in Chinese) 杨素萍, 林志华, 崔小华, 连建科, 赵春贵, 曲音波. 不产氧光合细菌的分类学进展. 微生物学报, 2008, 48(11): 1562-1566. DOI:10.3321/j.issn:0001-6209.2008.11.023 |
| [27] | Yu JL, Xia JJ, Ma QL, Zhang C, Zhao J, Tanggood X, Yang YF. Soil particle and moisture-related factors determine landward distribution of bacterial communities in a lateral riverside continuum of the Xilin River Basin. Soil Ecology Letters, 2021, 3(4): 303-312. DOI:10.1007/s42832-021-0106-2 |
| [28] | 鮑士旦. 土壤农化分析. 第3版. 北京: 中国农业出版社, 2002. |
| [29] | Yang C, Sun J, Chen Y, Wu J, Wang Y. Linkage between water soluble organic matter and bacterial community in sediment from a shallow, eutrophic lake, Lake Chaohu, China. Journal of Environmental Sciences, 2020, 98: 39-46. DOI:10.1016/j.jes.2020.05.023 |
| [30] | De'Ath G. Multivariate regression trees: a new technique for modeling species-environment relationships. Ecology, 2002, 83(4): 1105-1117. |
| [31] | Rosseel Y. Lavaan: an R package for structural equation modeling. Journal of Statistical Software, 2012, 48: 1-36. |
| [32] | Gutiérrez-Preciado A, Saghaï A, Moreira D, Zivanovic Y, Deschamps P, López-García P. Functional shifts in microbial mats recapitulate early Earth metabolic transitions. Nature Ecology & Evolution, 2018, 2(11): 1700-1708. |
| [33] | Sofrová D. Sulfur metabolism in phototrophic organisms. Biologia Plantarum, 2009, 53(3): 538. |
| [34] | Ha PT, Lindemann SR, Shi L, Dohnalkova AC, Fredrickson JK, Madigan MT, Beyenal H. Syntrophic anaerobic photosynthesis via direct inter species electron transfer. Nature Communications, 2017, 8: 13924. DOI:10.1038/ncomms13924 |
| [35] | Chung J, Shin S, Oh J. Characterization of a microbial community capable of reducing perchlorate and nitrate in high salinity. Biotechnology Letters, 2009, 31(7): 959-966. DOI:10.1007/s10529-009-9960-1 |
| [36] |
Dai LX, Xu Y, Zhang GC, Shi XL, Qin FF, Ding H, Zhang ZM. Response of rhizosphere bacterial community diversity to salt stress in peanut. Acta Agronomica Sinica, 2021, 47(8): 1581-1592.
(in Chinese) 戴良香, 徐扬, 张冠初, 史晓龙, 秦斐斐, 丁红, 张智猛. 花生根际土壤细菌群落多样性对盐胁迫的响应. 作物学报, 2021, 47(8): 1581-1592. |
| [37] | Zhang GI, Hwang CY, Cho BC. Thalassobaculum litoreum gen. nov., sp. nov., a member of the family Rhodospirillaceae isolated from coastal seawater. International Journal of Systematic & Evolutionary Microbiology, 2008, 58(2): 479-485. |
| [38] | Kim JH, Konkit M, Yoon JH, Kim W. Limibacillus halophilus gen. nov., sp. nov., a moderately halophilic bacterium in the family Rhodospirillaceae isolated from reclaimed land. International Journal of Systematic and Evolutionary Microbiology, 2015, 65: 3155-3161. DOI:10.1099/ijs.0.000346 |
| [39] | Simon M, Scheuner C, Meier-Kolthoff JP, Brinkhoff T, Wagner-Döbler I, Ulbrich M, Klenk HP, Schomburg D, Petersen J, Göker M. Phylogenomics of Rhodobacteraceae reveals evolutionary adaptation to marine and non-marine habitats. The ISME Journal, 2017, 11(6): 1483-1499. DOI:10.1038/ismej.2016.198 |
| [40] | 崔小华. 嗜盐紫色硫细菌耐盐机制与光合色素研究. 山西大学博士学位论文, 2013. |
| [41] | Jehlička J, Oren A, Vítek P. Use of Raman spectroscopy for identification of compatible solutes in halophilic bacteria. Extremophiles, 2012, 16(3): 507-514. DOI:10.1007/s00792-012-0450-3 |
| [42] | Igeno MI, Moral C, Castillo F, Caballero FJ. Halotolerance of the phototrophic bacterium Rhodobacter capsulatus E1F1 is dependent on the nitrogen source. Applied & Environmental Microbiology, 1995, 61(8): 2970-2975. |
| [43] | Boncompagni E, Østerås, Magne, Poggi MC, Rudulier D. Occurrence of choline and glycine betaine uptake and metabolism in the family Rhizobiaceae and their roles in osmoprotection. Applied & Environmental Microbiology, 1999, 65(5): 2072-2077. |
| [44] | Táncsics A, Szalay AR, Farkas M, Benedek T, Szoboszlay S, Szabó I, Lueders T. Stable isotope probing of hypoxic toluene degradation at the Siklós aquifer reveals prominent role of Rhodocyclaceae. FEMS Microbiology Ecology, 2018, 94(6): fiy088. |
| [45] | Sun X, Cao X, Zhao D, Zeng J, Huang R, Duan M, Yu Z. The pattern of sedimentary bacterial communities varies with latitude within a large eutrophic lake. Limnologica-Ecology and Management of Inland Waters, 2021, 87(43): 125860. |
| [46] | Gomila M, Pinhassi J, Falsen E, Moore ERB, Lalucat J. Kinneretia asaccharophila gen. nov., sp. nov., isolated from a freshwater lake, a member of the Rubrivivax branch of the family Comamonadaceae. International Journal of Systematic and Evolutionary Microbiology, 2010, 60(Pt 4): 809–814. |
| [47] | Chu LB, Wang JL. Denitrification performance and biofilm characteristics using biodegradable polymers PCL as carriers and carbon source. Chemosphere, 2013, 91(9): 1310-1316. DOI:10.1016/j.chemosphere.2013.02.064 |
| [48] | Fahrbach M, Kuever J, Meinke R, Kämpfer P, Hollender J. Denitratisoma oestradiolicum gen. nov., sp. nov., a 17 β-oestradiol-degrading, denitrifying Betaproteobacterium. International Journal of Systematic & Evolutionary Microbiology, 2006, 56(7): 1547-1552. |
| [49] | Lv YF, Pan JJ, Huo TR, Li JQ, Liu ST. Enhance the treatment of low strength wastewater at low temperature with the coexistence system of AnAOB and heterotrophic bacteria. Performance and bacterial community. Science of the Total Environment, 2020, 714: 136799-136799. |
| [50] | Yuan X, Knelman JE, Wang D, Goebl A, Gasarch E, Seastedt TR. Patterns of soil bacterial richness and composition tied to plant richness, soil nitrogen, and soil acidity in alpine tundra. Arctic, Antarctic, and Alpine Research, 2017, 49(3): 441-453. DOI:10.1657/AAAR0016-050 |
| [51] | Itakura M, Saeki K, Omori H, Yokoyama T, Kaneko T, Tabata S, Ohwada T, Tajima S, Uchiumi T, Honnma K, Fujita K, Iwata H, Saeki Y, Hara Y, Ikeda S, Eda SM, Mitsui H, Minamisawa K. Genomic comparison of Bradyrhizobium japonicum strains with different symbiotic nitrogen-fixing capabilities and other Bradyrhizobiaceae members. The ISME Journal, 2009, 3(3): 326-339. DOI:10.1038/ismej.2008.88 |
| [52] | Trüper HG, Pfennig N. Characterization and identification of the anoxygenic phototrophic bacteria. The Prokaryotes, 1981: 299-312. |
 2022, Vol. 62
2022, Vol. 62