
中国科学院微生物研究所,中国微生物学会
文章信息
- 陈龙, 金阿南, 马香娟, 吴镝, 冯华军. 2022
- CHEN Long, JIN Anan, MA Xiangjuan, WU Di, FENG Huajun.
- 微生物高盐渗透适应策略及其耐盐强化研究进展
- Research progress on osmotic pressure adaptation strategy and salt tolerance enhancement of microorganisms under high salinity environment
- 微生物学报, 62(9): 3306-3317
- Acta Microbiologica Sinica, 62(9): 3306-3317
-
文章历史
- 收稿日期:2022-01-25
- 修回日期:2022-02-08
- 网络出版日期:2022-05-16
2. 浙江工商大学废物低碳循环与绿色发展国际合作基地, 浙江 杭州 310012;
3. 比利时根特大学生物工程学院, 比利时 根特 9000
2. International Science and Technology Cooperation Platform for Low-Carbon Recycling of Waste and Green Development, Zhejiang Gongshang University, Hangzhou 310012, Zhejiang, China;
3. Faculty of Bioscience engineering, Ghent University, Ghent 9000, Belgium
2017年我国废水产生总量约为690亿t,并以每年2%的速率增长[1]。高盐废水产生量一般约占废水总量的5%,依此估算2022年我国高盐废水产生量高达38.2亿t[2]。高盐废水的来源可分为2大类:一类为海水(盐度2.5–3.5wt%)的直接利用,如中国香港地区因缺乏淡水资源,采用海水直接冲厕;另一类为工业高盐废水(盐度3.5–20.0wt%)的排放,包括石油、印染、化工等行业排放的废水。主流观点认为,盐度(以NaCl计)超过1.0wt%的废水为高盐废水,但也存在其他划分标准[3]。Shi等将废水分为含盐废水(盐度大于1.0wt%)和高盐废水(盐度大于3.5wt%)[4];Pernetti等将废水分为含盐废水(盐度0–1.0wt%)、高盐废水(盐度1.0–3.5wt%)和超高盐废水(盐度大于3.5wt%)[5]。
生物法和物化法是常见的废水处理技术。常规微生物在高盐环境下会因渗透失衡而丧失细胞活性,因此物化法是当前高盐废水的主要处理方法。但其在实际应用过程中存在诸多痛点:电解、芬顿等高级氧化技术的处理成本远高于生物处理技术,且对于低浓度有机物去除效果不明显[6];蒸馏、浓缩等物理技术的固体盐产物被判定为危险废物,后续处理困难[7]。因此,亟需开发经济、高效、可靠的生物处理技术来解决高盐废水处理难题。
高盐废水易对微生物正常代谢产生抑制作用,而耐盐微生物和嗜盐微生物因具有特定的高渗透压适应策略(以下简称“高渗适应策略”)得以在高盐环境下维持正常生理代谢。但由于嗜盐微生物生长条件苛刻、功能微生物种类稀缺,因此在盐度较高(3wt%左右)的废水处理中耐盐微生物的普适性更强、挖掘空间更大。Kurt-Kizildogan等、Mokashe等和Tan等都曾对高盐环境中微生物进行综述,此类研究对微生物的分类较为模糊,对其高渗适应策略的总结不全面,对相关耐盐强化调控技术的探究较少[8–10]。因此,本文系统总结了高盐环境微生物的分类标准,阐述了微生物高渗适应策略的研究现状,探讨了部分耐盐强化调控技术的研发进展,并对未来研究方向进行了展望。
1 高盐环境中的微生物在高盐环境中生存的微生物可分为耐盐微生物(halotolerant microorganism)和嗜盐微生物(halophilic microorganism)。Uratani等认为耐盐微生物能够在不同盐度下兼性生长,嗜盐微生物只能在特定盐度范围内专性生长[11]。罗玲等认为耐盐微生物在无盐条件下生长最佳,而嗜盐微生物的生长离不开高盐环境[2]。由此可见,目前对于嗜盐和耐盐微生物的定义并没有清晰统一的界定标准[12]。结合已有报道,本文提出嗜盐微生物是指在最适盐度范围内生存最好并进行专性生长的一类微生物,盐是其生长的必要条件;耐盐微生物是指可耐受较低盐度并在相对较大盐度范围内兼性生长的一类微生物,盐不是其生长的必要条件。
常用的微生物分类方法仅可对部分极端嗜盐微生物菌种进行确认,包括测定微生物16S rRNA/DNA序列、DNA的GC含量以及对微生物进行DNA杂交分析等[13]。目前大多数研究是根据微生物的最适盐度范围进行分类,以Kushner团队提出的分类方法为主流[14–18]。此外,Ray等还发现嗜盐和耐盐微生物对于4, 6-二甲基嘧啶-2-硫醇(4, 6-DMPT)的敏感程度不同,提出该物质有助于在体外进一步对2类微生物进行区分[19]。
但现有大多数文献对2类微生物耐盐范围的区分较为混乱,没有统一的标准。Kushner在1993年出版的The Biology of Halophilic Bacteria一书中根据微生物的耐盐能力进行区分[14]。Lay等和Mokashe等依据耐盐、嗜盐微生物对盐度的依赖性也进行了分类(表 1)[9, 20]。另外,根据BacDive网站统计,82 892种菌株的平均最佳盐度为3.03wt%,证明在3wt%盐度附近的微生物数量最多、种类最丰富。
| Microbial species | Optimum salinity range (in NaCl)/wt% | ||
| Reference [14] | Reference [20] | Reference [9] | |
| Halotolerant microorganism | <1.17 | <1.00 | <1.10 |
| Slight halophile microorganism | 1.17–2.90 | 1.00–3.00 | 1.10–2.75 |
| Moderate halophile microorganism | 2.90–14.60 | 3.00–15.00 | 3.30–11.00 |
| Borderline extreme halophile microorganism | 14.60–23.40 | 10.50–16.50 | |
| Extreme halophile microorganism | 23.40–34.50 | >15.00 | 16.50–25.90 |
嗜盐微生物生长条件苛刻,在非最适盐度下生命力较脆弱,易丧失生物活性;并且嗜盐微生物中古菌占据比例较大,功能微生物种类稀缺,可挖掘空间较小[21]。反观耐盐微生物生存范围广,可耐受一定浓度的盐溶液,在非最适盐度环境下处于胁迫状态,但仍能进行正常的生长代谢活动[10]。综上,耐盐微生物在盐度较高(3wt%左右)的环境下普适性更强,实际应用场景更为丰富[22–23]。
2 高盐环境微生物渗透压适应策略现有文献对耐盐微生物和嗜盐微生物分类较为模糊,且研究对象多为混菌体系,难以区分在高盐环境下到底哪类微生物起主要作用,而以纯菌为研究对象的多以嗜盐微生物为主,对于耐盐微生物的研究较少[10, 24]。鉴于此,本文并未严格区分2类微生物的高渗适应策略,而是系统阐述了微生物在高盐环境下的盐溶、胞内小分子相容溶质积累、蛋白质稳定和细胞表面稳定等高渗适应策略。
2.1 盐溶策略盐溶策略(salt-in)是极端嗜盐杆菌的典型耐盐策略,同时还存在于厌氧嗜盐真菌和红盐杆菌中[25–26]。研究发现嗜盐微生物的Na+输出基因和K+吸收基因具有较高的表达能力,其中Na+/H+逆向转运蛋白可在ATP驱动下将胞内多余的Na+排出细胞。Edbeib等已对Na+的相关高渗适应策略进行了详细的总结,本文不做过多介绍[27]。
K+转运蛋白多为单向转运,通过胞内累积高浓度K+来平衡内外渗透压[28]。并不是所有累积的K+都具有渗透活性,一部分K+需要平衡细胞质大分子的负电荷,只有平衡其他小分子负电荷的K+才对细胞起渗透保护作用[29]。细胞中维持K+内稳态的系统主要有Kdp、Trk和Tup等系统。(1) Kdp系统。该系统通过一种P型ATP酶的作用分解ATP产生能量进而吸收K+。Strahl等发现嗜盐菌极端嗜盐古生菌(Halobacterium salinarum) R1通过表达K+转运蛋白KdpFABC P型ATP酶来抵抗高盐环境[30]。(2) Trk系统。Trk系统由TrkH或TrkG的跨膜蛋白组成,当介质中K+浓度大于1 mmol/L时,Trk系统起主要作用。(3) Kup系统(也称作TrkD系统)。Kup系统是一个低K+吸引力的系统,主要起辅助作用[29]。正是由于上述Kdp系统等多种胞内K+维稳系统的调节作用,微生物才能在高盐环境下通过盐溶策略维持细胞良好活性。
Speth等发现厌氧氨氧化细菌(anaerobic ammonium oxidation,Anammox)可以积累K+或胞内小分子相容溶质,该物质在高盐条件下能够保持细菌的渗透平衡[31]。Vaidya等经检测发现在高盐环境下极端嗜盐菌胞内K+浓度较高,而大部分耐盐菌胞内K+浓度较低(约为极端嗜盐菌的1/10),但其中一株耐盐菌SS8的胞内K+浓度较高[32]。这表明盐溶策略主要存在于嗜盐微生物中,但也可能在极少数耐盐微生物中发挥作用。此外,最新研究表明,胞内第二信使在环境胁迫下将对离子吸收起调节作用,如cyclic-di-AMP和cyclic-di-GMP。Cui等通过研究发现在山梨醇诱导的渗透胁迫下,Synechocystis sp. PCC 6803中cyclic-di-AMP增长3倍,在蓝细菌(Fremyella diplosiphon)中增长2倍[33]。进一步研究发现,c-di-AMP与KdpD (组氨酸激酶)结合抑制了盐胁迫下KdpFABC的上调,表明c-di-AMP是葡萄球菌(Staphylococcus aureus)胞内K+吸收的负调节因子。关于第二信使的相关研究较少,仍处于研究探索阶段。
2.2 胞内小分子相容溶质积累策略相容性溶质具有分子量低、水溶性强、毒性弱等特点,可在生物细胞内高浓度积聚以维持细胞渗透平衡。微生物可通过胞内合成或从周围介质中直接获取进而积累胞内小分子相容溶质,包括甘油、蔗糖、外泌素和甘氨酸甜菜碱等。甘油是分子量最小的有机渗透溶质,由于该物质的高渗透性导致其在原核微生物中几乎无法存留,只存在于部分真核微生物中[34];甘氨酸甜菜碱是分子量第二小的有机渗透物质,是众多微生物在高渗环境下积累的主要胞内小分子相容溶质[34];最近还发现了盐胁迫下蔗糖积累的新策略[35]。
崔小华研究了海洋着色菌Marichromatium sp. 283-I的生物学特性及高渗适应策略,经HPLC检测发现菌株胞内积累高含量的甜菜碱,且随着盐浓度升高甜菜碱含量也逐渐升高[36]。Park等通过实验验证盐单胞菌Halomonas socia CKY01可在胞内合成外泌碱和脯氨酸等小分子溶质来耐受盐胁迫[37]。杨重等对2株耐盐菌株葡萄球菌Staphylococcus sp. YZ-1和芽孢杆菌Bacillus cereus CC-1进行了基因组序列分析,发现都含有编码甘氨酸甜菜碱转运蛋白基因[38]。邱凯旋等探究了石油降解菌HX-2菌株的甜菜碱合成基因(BetB)、转运蛋白基因(H0、H1、H3和H5)及其在高盐条件下的表达情况,结果显示甜菜碱转运蛋白基因的表达与盐度及甜菜碱投加量呈正相关性[39]。这些胞内小分子相容溶质是极性和可溶的两性离子,也被称作强水结构形成剂,在蛋白质分子没有变性的情况下发挥稳定作用[40]。大多数耐盐和轻度、中度嗜盐生物,包括无氧光合细菌、需氧异养细菌、蓝藻和产甲烷菌,普遍通过积累或合成胞内小分子相容溶质来维持细胞渗透平衡[8]。研究表明,使用胞内小分子相容溶质积累策略的微生物通常比使用盐溶策略的微生物更灵活,更容易适应压力或盐度突变。当受到低浓度盐度冲击时,胞内过量的小分子相容溶质可通过机械敏感通道排出,或在细胞内转化为非活性状态。在盐度突然增加后,无机离子可以在胞内短暂地积累,然后被新合成的有机溶质所取代。此外,这些小分子相容溶质还可作为稳定剂,维持细胞内各类大分子的活性构象,以适应极端条件。经上述讨论后发现,虽然胞内小分子相容溶质的摄取、合成所需能量高于盐溶策略消耗的能量,但胞内小分子相容溶质积累策略却被微生物普遍采用,这表明该策略的适用性更强,平衡渗透压效果更好。
2.3 蛋白质稳定策略高盐溶液会增加水的表面张力,导致微生物的蛋白质和溶液中的盐离子之间进行水化竞争。盐离子从蛋白质表面清除一层重要的水分子,导致普通微生物的蛋白质变性、失去活性构象[41]。而耐盐和嗜盐微生物通常需要特定的盐度才能表现出蛋白质/酶的溶解性、稳定性和催化性[42]。
嗜盐微生物蛋白酶在高盐度环境中表现出最佳活性,大多属于丝氨酸蛋白酶组;耐盐微生物蛋白酶无盐依赖性,主要由金属蛋白酶组和丝氨酸蛋白酶组构成。丝氨酸内蛋白酶(E.C.3.4.21)以丝氨酸作为活性中心参与催化过程,由丝氨酸(Ser)、组氨酸(His)和天冬氨酸(Asp)组成,它们通过三级结构组合在催化位点上,通常被称为“催化三联体” (catalytic triad)[9, 43]。金属蛋白酶以金属离子作为活性催化中心,并作为辅助因子与酶紧密结合。这些金属离子还可以提升酶结构的稳定性,共同形成活性构象[9]。Soppa以耐盐酶DehHX为例发现以下特点:(1) GC含量高;(2) 酸性残留物含量高;(3) 碱性和疏水残留物含量低;(4) 表面负电荷高[44]。酸性残基位于蛋白质表面,参与形成一个强大的水合壳(hydration shell),可以抵抗蛋白质聚集体的形成。同时羧酸离子所产生的大量负电荷表面残基有助于提高DehHX的稳定性[45]。进一步研究发现,耐盐和嗜盐微生物蛋白质的氨基酸组成与普通微生物蛋白质的氨基酸组成相比具有以下特点:(1) 多出近20%的酸性氨基酸残基(如天冬氨酸和谷氨酸);(2) 赖氨酸残基更少;(3) 与非嗜盐酶相比,小疏水残基(丙氨酸和甘氨酸)和极性残基(丝氨酸和苏氨酸)数量增加[9, 46]。这些特点均有助于微生物在高盐环境下平衡渗透压,降低细胞表面的疏水性,提升细胞稳定性,并促进高盐条件下的细胞水化作用。
2.4 细胞表面稳定策略上述各种稳定策略均在细胞质中进行,暴露在高盐环境的细胞表面也存在相应稳定策略。随着盐度的增加,嗜盐菌的细胞膜阴离子磷脂的比例逐渐提高,产生额外的膜表面电荷,有助于维持细胞质膜的水化状态。同时胞外聚合物(extracellular polymeric substances,EPS)数量快速增加,通过改变表面电荷、疏水性等物理化学特性,增强微生物的主动运输和扩散活动,因此,EPS对于污泥颗粒化、生物膜形成和结构完整性的维护至关重要[47–51]。
Hong等发现随着盐度从0 g/L提高到35 g/L过程中,EPS含量也随之增加,由13.39 mg/(g MLSS)增长到39.98 mg/(g MLSS)[52]。Liu等发现不能产生EPS的细菌相较于产生EPS的细菌细胞内含有更高的Na+浓度,并且产生EPS的细菌可能具有更强的耐盐性[53]。最新研究发现,微生物EPS的增加可能与微生物的群体感应(quorum sensing,QS)相关。Li等发现在盐度2wt%和4wt%条件下活性污泥处理有机物能力显著降低,添加酰基高丝氨酸内酯(acyl-homoserine lactone,AHL)介导的群体感应信号分子混合物(C6-HSL、C8-HSL和3OC8-HSL各0.1 μmol/L)可促进微生物EPS的分泌,促进作用在盐度2wt%条件下明显,但在盐度4wt%条件下不显著[54]。Zhu等发现高浓度的信号分子AHLs促进厌氧氨氧化细菌产生更多的EPS,提高其对盐胁迫的耐受性[55]。Gu等发现外源补充AI-2可以促进EPS的产生、并改变与多糖合成的相关基因lamC和ftsH,最终促进植物乳杆菌(Lactobacillus plantarum)生物膜的形成并提升环境耐受性[56]。
综上,本文讨论了高盐环境微生物的4种高渗适应策略,发现在高盐环境下耐盐微生物存在高渗透压适应多策略协同现象,如SS8同时采用盐溶策略和胞内小分子相容溶质积累策略,因此,筛选出具有多种高渗适应策略的耐盐微生物意义重大。未来研究应探明上述策略之间是否存在协同或抑制作用,是否还存在其他未知的高渗适应策略等,进而最大限度地利用微生物耐盐能力和污染物降解能力。
3 微生物耐盐强化调控技术由表 1可知,耐盐微生物在较高盐度条件下凭借胞内小分子相容溶质积累策略等部分高渗适应策略维持细胞渗透压平衡,但生物活性较低;嗜盐微生物在高盐条件下因具有盐溶策略等高渗适应策略而表现出良好活性[9, 14, 20, 57]。已有研究证实,驯化是一种提高微生物极端环境耐受性的有效方法[58]。高盐驯化是指通过逐渐提升环境盐度使耐盐微生物存活下来并成为优势微生物的过程。虽然高盐驯化已被证实是可行的,但在实验室条件下对微生物进行连续不间断培养通常需要较长时间才能获得理想的突变基因。为符合实际工程应用要求,亟需开发出一种快速、高效、稳定的微生物耐盐强化调控技术。最新研究发现,电、光、磁调控技术可有效加速高盐环境微生物的驯化过程,还能强化微生物的污染去除能力,经调控后的耐盐微生物活性较高,但具体强化调控的高渗适应策略尚不明确[59–67]。微生物高渗适应机制及耐盐强化技术示意图详见图 1。
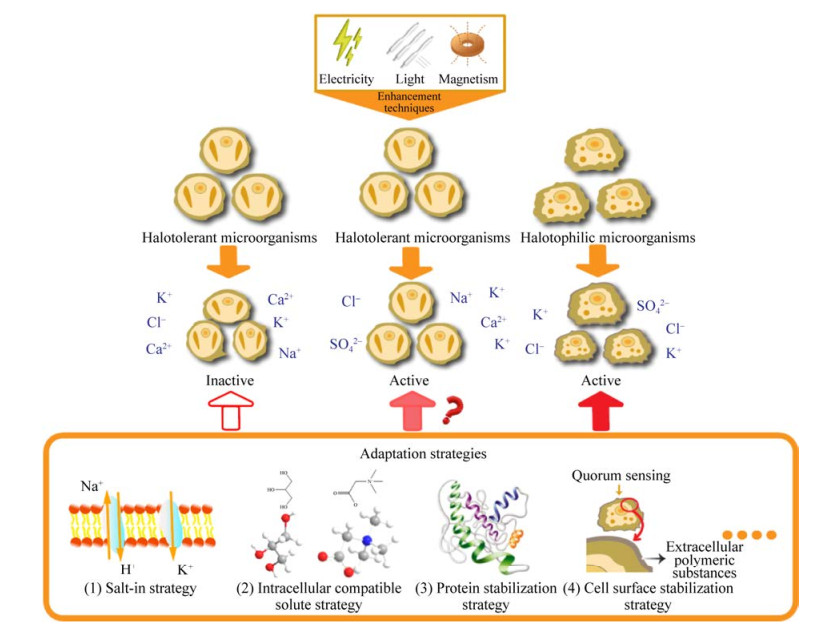
|
| 图 1 微生物高盐渗透适应策略及其耐盐强化技术 Figure 1 High salinity osmotic adaptation strategies and salt tolerance enhancement techniques of microorganisms. |
3.1 电强化调控技术
电强化调控技术是通过电子传递方式将能量提供给环境微生物,促进其自身合成可溶性蛋白和多糖,提高关键酶活性,有助于微生物快速适应高盐环境[59]。为探究电强化调控技术是否可以提升高盐环境下微生物的耐受能力,Cardenas-Robles等利用生物电化学系统(bioelectrochemical system,BES)处理高盐染料废水,并发现在电场作用下的微生物生长速度显著提升,污泥浓度也提升了约40%[68]。Zhang等利用电辅助厌氧技术处理高盐废水,发现电场的存在增强了挥发性脂肪酸的生物降解,并通过PCR-DGGE的分析表明电强化调控技术在处理过程可有效富集耐盐微生物[69]。本课题组前期对电强化调控技术展开了研究,发现在40 g/L NaCl的高盐环境下,微生物处理对氟硝基苯(p-fluoronitrobenzene,p-FNB)能力几乎完全丧失,而在BES体系中相同时间内p-FNB去除率达100%,氟离子累积浓度为(0.149±0.020) mmol/L。因此,连续的电强化调控技术提升了BES中微生物的高渗透压适应能力并加强了目标污染物的降解能力。为进一步了解电强化调控策略,本课题组对电强化调控前后的微生物离子含量和蛋白/多糖比值进行测定,发现经电强化调控后的微生物胞内K+浓度提高了4.78倍,而Na+的浓度只上升了1.70倍。此外,蛋白/多糖的比值也从3.09升高到7.79[59]。但目前此类研究多以混菌体系为主,研究目标集中在污染物去除效果的验证。电强化调控技术到底是通过提高混菌体系中耐盐微生物的高渗适应能力还是加速混菌体系中嗜盐微生物的富集尚未有明确报道。因此,在未来研究电强化调控机制时应进行纯菌试验验证,进一步开展系统、深入的机制研究对实际应用意义重大。
3.2 光强化调控技术利用光强化调控技术(如UV光)提升微生物高渗适应能力是近年来的研究热点。虽然紫外线照射会引起微生物诱变DNA损伤,进而导致生物失活,但研究发现,将UV光强度控制在合适范围,即可在较高盐度条件下提高污染物去除率[60–64]。Hartke等发现乳酸乳杆菌在254 nm紫外线照射下暴露30 min将产生热应激蛋白(如GroEL和GroES),同时可能产生交叉保护效应,有效提升菌种高渗适应能力[70]。Wang等从南极苔藓(Pohlia nutans)中分离到一个CPD光解酶基因PnPHR1,并发现UV-B辐射可提高其表达水平[62]。该基因通过增强根际微生物胞内Na+/H+逆向转运蛋白基因(NHX)表达、高亲和力钾转运蛋白(HKT)表达以及胞内脯氨酸合成,进而提升其对UV-B辐射和盐胁迫的耐性。张琳等分离到一株中度嗜盐菌Halomonas TTW4,通过紫外诱变获得突变株UV-1。经检测发现UV-1胞内甜菜碱含量最高可达190 μg/mg,而盐敏感突变株胞内甜菜碱含量在150 μg/mg以下,最低的只有110 μg/mg[71]。综上,UV光照射可产生交叉保护效应并增加微生物胞内甜菜碱等胞内小分子相容溶质含量,但关于光照强化调控微生物高渗适应能力的相关报道较少,相关机制仍不够清晰。
3.3 磁强化调控技术静态磁场(static magnetic field,SMF)可以降低扩散阻力对酶反应动力学的影响,加速酶接近底物并与底物重组,最终体现在微生物数量的增加以及处理效率的提升[65–67]。Niu等发现SMF可以促进细胞质膜产生更多的不饱和脂肪酸,并推测SMF可能加速或改变微生物调控过程而增强对高盐环境的耐受性[72–73]。Shao等研究了中强度SMF对海洋微生物群落在不同盐度条件下进行偶氮染料脱色的影响。在恒定磁场强度作用下,300 mg/L酸性红B (acid red B,ARB)在0、30、50 g/L的盐度条件下的脱色率分别为94.6%–98.9%、92.1%–98.9%和68.7%[74]。Wang等也进行了类似实验,在30、50、70 g/L NaCl的情况下,以不进行磁场调控和施加24.6 mT的SMF进行12 h脱色,结果发现磁场调控后的脱色率较无磁场对照组提高了约138%,同时磁场对酵母细胞的生长还有轻微的促进作用[75]。通过上述研究发现,在磁场强化调控作用下微生物的脱色效率得到提升,并且在盐度较高的条件下仍可以保持理想脱色率。但当盐度超出一定范围后,微生物脱色能力将迅速丧失,表现为ARB脱色率明显下降。因此,在一定磁场调控作用下,需要控制合适的盐度条件,进而最大限度地发挥高盐废水中的微生物去污潜能。
电、光、磁强化调控技术均可在一定程度上提升高盐环境微生物的高渗适应能力以及污染物去除能力。考虑到光、磁强化调控技术的可控性较差且目前机理研究完善度较低,而电强化调控技术相对可控且具有一定的工程应用前景,因此,电强化调控技术或许是未来高盐废水生物处理的重点研发方向,但具体调控机制还有待进一步深入探究。
4 结论与展望目前,利用微生物处理高盐废水已取得一定进展,本文在分子水平上梳理总结耐盐微生物和嗜盐微生物的高渗适应策略以及耐盐强化调控技术,但许多强化调控技术机制仍亟待探明,为未来实际工程应用提供理论依据:
(1) 针对盐度在3wt%左右的废水,未来研究重点可放在将普通微生物驯化为耐盐微生物上。这样既可保留微生物的功能基因多样性,又可提升相应的耐盐能力。
(2) 迫切需要建立一个在盐胁迫环境中调控微生物的基因、蛋白质和代谢产物的相互作用网络,深入了解微生物的高渗适应策略,并扩大微生物的盐度适应范围,以便有效适应水质水量波动大的高盐工业废水处理需求。
(3) 相较于光、磁强化调控技术的可控性较强、且成熟度不高的特点,电强化调控技术的可控性较佳且具有良好的实际应用前景,但其强化调控技术的详细机制有待进一步探明。
| [1] | Le Borgne S, Paniagua D, Vazquez-Duhalt R. Biodegradation of organic pollutants by halophilic bacteria and Archaea. Journal of Molecular Microbiology and Biotechnology, 2008, 15(2/3): 74-92. |
| [2] |
Luo L, Yuan Y, Hao HC, Lu LX, Tang LW, Zhong CM. Research progress on treatment of high-salt wastewater by MBR with halotolerant microorganisms. Technology of Water Treatment, 2021, 47(11): 13-17.
(in Chinese) 罗玲, 袁野, 郝会超, 陆柳鲜, 唐林旺, 钟常明. MBR高盐废水处理中耐盐微生物研究进展. 水处理技术, 2021, 47(11): 13-17. |
| [3] | 鄢忠森. 膜蒸馏处理高盐废水过程中膜污染和膜润湿及控制. 哈尔滨工业大学博士学位论文, 2019. |
| [4] | Shi K, Zhou WZ, Zhao HX, Zhang YZ. Performance of halophilic marine bacteria inocula on nutrient removal from hypersaline wastewater in an intermittently aerated biological filter. Bioresource Technology, 2012, 113: 280-287. DOI:10.1016/j.biortech.2012.01.117 |
| [5] | Pernetti M, Palma LD. Experimental evaluation of inhibition effects of saline wastewater on activated sludge. Environmental Technology, 2005, 26(6): 695-704. DOI:10.1080/09593330.2001.9619509 |
| [6] | Srivastava A, Parida VK, Majumder A, Gupta B, Gupta AK. Treatment of saline wastewater using physicochemical, biological, and hybrid processes: insights into inhibition mechanisms, treatment efficiencies and performance enhancement. Journal of Environmental Chemical Engineering, 2021, 9(4): 105775. DOI:10.1016/j.jece.2021.105775 |
| [7] | Shi J, Huang W, Han H, Xu C. Review on treatment technology of salt wastewater in coal chemical industry of China. Desalination, 2020, 493: 114640. DOI:10.1016/j.desal.2020.114640 |
| [8] | Kurt-Kizildogan A, Abanoz B, Okay S. Global transcriptome analysis of Halolamina sp. to decipher the salt tolerance in extremely halophilic archaea. Gene, 2017, 601: 56-64. DOI:10.1016/j.gene.2016.11.042 |
| [9] | Mokashe N, Chaudhari B, Patil U. Operative utility of salt-stable proteases of halophilic and halotolerant bacteria in the biotechnology sector. International Journal of Biological Macromolecules, 2018, 117: 493-522. DOI:10.1016/j.ijbiomac.2018.05.217 |
| [10] | Tan X, Acquah I, Liu HZ, Li WG, Tan SW. A critical review on saline wastewater treatment by membrane bioreactor (MBR) from a microbial perspective. Chemosphere, 2019, 220: 1150-1162. DOI:10.1016/j.chemosphere.2019.01.027 |
| [11] | Uratani JM, Kumaraswamy R, Rodríguez J. A systematic strain selection approach for halotolerant and halophilic bioprocess development: a review. Extremophiles, 2014, 18(4): 629-639. DOI:10.1007/s00792-014-0659-4 |
| [12] | Borowitzka MA. The 'stress' concept in microalgal biology—homeostasis, acclimation and adaptation. Journal of Applied Phycology, 2018, 30(5): 2815-2825. DOI:10.1007/s10811-018-1399-0 |
| [13] | Cui HL, Zhou PJ, Oren A, Liu SJ. Intraspecific polymorphism of 16S rRNA genes in two halophilic archaeal genera, Haloarcula and Halomicrobium. Extremophiles, 2009, 13(1): 31-37. DOI:10.1007/s00792-008-0194-2 |
| [14] | Kushner DJ. Growth and nutrition of halophilic bacteria//Russel H. Vreeland, Lawrence I. Hochstein. The Biology of Halophilic Bacteria. Boca Raton, Florida: CRC Press, 1993: 87–103. |
| [15] | Seck EH, Dufour JC, Raoult D, Lagier JC. Halophilic & halotolerant prokaryotes in humans. Future Microbiology, 2018, 13: 799-812. DOI:10.2217/fmb-2017-0237 |
| [16] | 许瑶. 高盐环境原核生物多样性及一株嗜盐古菌的多相分类学研究. 安徽师范大学硕士学位论文, 2019. |
| [17] |
Chen QH, Liu ZX, Peng QZ, Huang N, He JW, Zhang L, Li WJ, Chen YG. Diversity of halophilic and halotolerant bacteria isolated from non-saline soil collected from Xiaoxi National Natural Reserve, Hunan Province. Acta Microbiologica Sinica, 2010, 50(11): 1452-1459.
(in Chinese) 陈奇辉, 刘祝祥, 彭清忠, 黄苛, 贺建武, 张丽, 李文均, 陈义光. 小溪自然保护区非盐环境土壤中嗜盐和耐盐菌多样性. 微生物学报, 2010, 50(11): 1452-1459. |
| [18] | 丁舒心. 巴丹吉林沙漠湖泊两株嗜耐盐菌降解废水中对硝基酚特性的研究. 内蒙古大学硕士学位论文, 2020. |
| [19] | Ray R, Das AK, Dutta NK, Chakrabarty AN, Chaudhuri BN, Seth S, Dastidar SG. Potentiality of a new compound for in vitro differentiation between halophilic and non-halophilic vibrios. Indian Journal of Experimental Biology, 2002, 40(2): 220-222. |
| [20] | Lay WCL, Liu Y, Fane AG. Impacts of salinity on the performance of high retention membrane bioreactors for water reclamation: a review. Water Research, 2010, 44(1): 21-40. DOI:10.1016/j.watres.2009.09.026 |
| [21] | 王重阳. 嗜盐微生物对典型多环芳烃的降解及其分子机制的研究. 清华大学博士学位论文, 2018. |
| [22] |
Ma YY, Chen JC, Chen GQ. Halophilic microorganisms as microbial chassis: applications and prospects. Chemical Industry and Engineering Progress, 2021, 40(3): 1178-1186.
(in Chinese) 马悦原, 陈金春, 陈国强. 嗜盐微生物底盘细胞: 应用和前景. 化工进展, 2021, 40(3): 1178-1186. |
| [23] |
Hu JC. Advances in the remediation of polycyclic aromatic hydrocarbons by halophilic and salttolerant microorganisms. Biological Chemical Engineering, 2020, 6(2): 139-142.
(in Chinese) 胡锦程. 嗜盐/耐盐微生物对多环芳烃污染修复的研究进展. 生物化工, 2020, 6(2): 139-142. DOI:10.3969/j.issn.2096-0387.2020.02.039 |
| [24] | Musa H, Kasim FH, Nagoor Gunny AA, Gopinath SCB. Salt-adapted moulds and yeasts: potentials in industrial and environmental biotechnology. Process Biochemistry, 2018, 69: 33-44. DOI:10.1016/j.procbio.2018.03.026 |
| [25] | Sleator RD, Hill C. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiology Reviews, 2002, 26(1): 49-71. DOI:10.1111/j.1574-6976.2002.tb00598.x |
| [26] | Gunde-Cimerman N, Plemenitaš A, Oren A. Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiology Reviews, 2018, 42(3): 353-375. DOI:10.1093/femsre/fuy009 |
| [27] | Edbeib MF, Wahab RA, Huyop F. Halophiles: biology, adaptation, and their role in decontamination of hypersaline environments. World Journal of Microbiology and Biotechnology, 2016, 32(8): 1-23. |
| [28] | Wang Q, Cao ZF, Liu Q, Zhang JY, Hu YB, Zhang J, Xu W, Kong Q, Yuan XC, Chen QF. Enhancement of COD removal in constructed wetlands treating saline wastewater: intertidal wetland sediment as a novel inoculation. Journal of Environmental Management, 2019, 249: 109398. DOI:10.1016/j.jenvman.2019.109398 |
| [29] | Lefebvre O, Moletta R. Treatment of organic pollution in industrial saline wastewater: a literature review. Water Research, 2006, 40(20): 3671-3682. DOI:10.1016/j.watres.2006.08.027 |
| [30] | Strahl H, Greie JC. The extremely halophilic archaeon Halobacterium salinarum R1 responds to potassium limitation by expression of the K+-transporting KdpFABC P-type ATPase and by a decrease in intracellular K+. Extremophiles, 2008, 12(6): 741-752. DOI:10.1007/s00792-008-0177-3 |
| [31] | Speth DR, Lagkouvardos I, Wang Y, Qian PY, Dutilh BE, Jetten MSM. Draft genome of Scalindua rubra, obtained from the interface above the discovery deep brine in the red sea, sheds light on potential salt adaptation strategies in anammox bacteria. Microbial Ecology, 2017, 74(1): 1-5. DOI:10.1007/s00248-017-0929-7 |
| [32] | Vaidya S, Dev K, Sourirajan A. Distinct osmoadaptation strategies in the strict halophilic and halotolerant bacteria isolated from lunsu salt water body of north west Himalayas. Current Microbiology, 2018, 75(7): 888-895. DOI:10.1007/s00284-018-1462-8 |
| [33] | Cui JY, Sun T, Chen L, Zhang WW. Engineering salt tolerance of photosynthetic cyanobacteria for seawater utilization. Biotechnology Advances, 2020, 43: 107578. DOI:10.1016/j.biotechadv.2020.107578 |
| [34] | Oren A. Thermodynamic limits to microbial life at high salt concentrations. Environmental Microbiology, 2011, 13(8): 1908-1923. DOI:10.1111/j.1462-2920.2010.02365.x |
| [35] | Liang Y, Zhang M, Wang M, Cuncun Q, Luo Q, Lu X. Freshwater cyanobacterium Synechococcus elongatus PCC 7942 adapts to salt stress environment via ion-induced enzymatic balance of compatible solute. Applied and Environmental Microbiology, 2020, 86(7): e02904-19. |
| [36] | 崔小华. 嗜盐紫色硫细菌耐盐机制与光合色素研究. 山西大学博士学位论文, 2013. |
| [37] | Park YL, Choi TR, Han YH, Song HS, Park JY, Bhatia SK, Gurav R, Choi KY, Kim YG, Yang YH. Effects of osmolytes on salt resistance of Halomonas socia CKY01 and identification of osmolytes-related genes by genome sequencing. Journal of Biotechnology, 2020, 322: 21-28. DOI:10.1016/j.jbiotec.2020.07.006 |
| [38] |
Yang Z, Xu TY, Liu YH, Zhou H. Cr(Ⅵ) detoxification characteristics of salt-tolerant Staphylococcus sp. YZ-1 and Bacillus cereus CC-1. Microbiology China, 2020, 47(1): 66-75.
(in Chinese) 杨重, 徐天怡, 刘奕含, 周豪. 耐盐菌Staphylococcus sp. YZ-1和Bacillus cereus CC-1的Cr(Ⅵ)脱毒特性与机理. 微生物学通报, 2020, 47(1): 66-75. |
| [39] |
Qiu KX, Wu S, Fu YY, Cao JC, Guan ZG, Guo P, Hu X, Huang L. Salt tolerance mechanism and betaine transport genes of a petroleum-degrading strain HX-2. Microbiology China, 2020, 47(6): 1685-1698.
(in Chinese) 邱凯旋, 吴思, 符悦悦, 曹家畅, 关志国, 郭鹏, 胡鑫, 黄磊. 石油降解菌HX-2耐盐机制及甜菜碱转运蛋白基因的研究. 微生物学通报, 2020, 47(6): 1685-1698. |
| [40] | Faria TQ, Lima JC, Bastos M, Maçanita AL, Santos H. Protein stabilization by osmolytes from hyperthermophiles: effect of mannosylglycerate on the thermal unfolding of recombinant nuclease a from staphylococcus aureus studied by picosecond time-resolved fluorescence and calorimetry. Journal of Biological Chemistry, 2004, 279(47): 48680-48691. DOI:10.1074/jbc.M408806200 |
| [41] | Sinha R, Khare SK. Effect of organic solvents on the structure and activity of moderately halophilic Bacillus sp. EMB9 protease. Extremophiles, 2014, 18(6): 1057-1066. DOI:10.1007/s00792-014-0683-4 |
| [42] | Mevarech M, Frolow F, Gloss LM. Halophilic enzymes: proteins with a grain of salt. Biophysical Chemistry, 2000, 86(2/3): 155-164. |
| [43] | Sana B. Marine Microbial Enzymes: Current Status and Future Prospects/ Kim S-K. Springer Handbook of Marine Biotechnology. Berlin, Heidelberg: Springer Berlin Heidelberg, 2015: 905–917. |
| [44] | Soppa J. From genomes to function: haloarchaea as model organisms. Microbiology, 2006, 152(Pt 3): 585-590. |
| [45] | Talon R, Coquelle N, Madern D, Girard E. An experimental point of view on hydration/solvation in halophilic proteins. Frontiers in Microbiology, 2014, 5: 66. |
| [46] | Premkumar L, Greenblatt HM, Bageshwar UK, Savchenko T, Gokhman I, Sussman JL, Zamir A. Three-dimensional structure of a halotolerant algal carbonic anhydrase predicts halotolerance of a mammalian homolog. Proceedings of the National Academy of Sciences, 2005, 102(21): 7493-7498. DOI:10.1073/pnas.0502829102 |
| [47] | Li J, Ye W, Wei D, Ngo HH, Guo W, Qiao Y, Xu W, Du B, Wei Q. System performance and microbial community succession in a partial nitrification biofilm reactor in response to salinity stress. Bioresource Technology, 2018, 270: 512-518. DOI:10.1016/j.biortech.2018.09.068 |
| [48] | Wang Y, Qin W, Jiang X, Ju F, Mao Y, Zhang A, Stahl DA, Zhang T. Seasonal prevalence of ammonia- oxidizing archaea in a full-scale municipal wastewater treatment plant treating saline wastewater revealed by a 6-year time-series analysis. Environmental Science & Technology, 2021, 55(4): 2662-2673. |
| [49] | He H, Chen Y, Li X, Cheng Y, Yang C, Zeng G. Influence of salinity on microorganisms in activated sludge processes: a review. International Biodeterioration & Biodegradation, 2017, 119: 520-527. |
| [50] | Ajao V, Bruning H, Rijnaarts H, Temmink H. Natural flocculants from fresh and saline wastewater: comparative properties and flocculation performances. Chemical Engineering Journal, 2018, 349: 622-632. DOI:10.1016/j.cej.2018.05.123 |
| [51] | Ontiveros-Valencia A, Tang YN, Krajmalnik-Brown R, Rittmann BE. Managing the interactions between sulfate- and perchlorate-reducing bacteria when using hydrogen-fed biofilms to treat a groundwater with a high perchlorate concentration. Water Research, 2014, 55: 215-224. DOI:10.1016/j.watres.2014.02.020 |
| [52] | Hong J, Li W, Lin B, Zhan M, Liu C, Chen BY. Deciphering the effect of salinity on the performance of submerged membrane bioreactor for aquaculture of bacterial community. Desalination, 2013, 316: 23-30. DOI:10.1016/j.desal.2013.01.015 |
| [53] | Liu XD, Luo YT, Li ZF, Wang JM, Wei GH. Role of exopolysaccharide in salt stress resistance and cell motility of Mesorhizobium alhagi CCNWXJ12–2T. Applied Microbiology and Biotechnology, 2017, 101(7): 2967-2978. DOI:10.1007/s00253-017-8114-y |
| [54] | Li C, Li W, Li H, Hou M, Wu X, Zhuang JL, Liu YD. The effect of quorum sensing on performance of salt-tolerance aerobic granular sludge: linking extracellular polymeric substances and microbial community. Biodegradation, 2019, 30(5/6): 447-456. |
| [55] | Zhu ZB, Zhang YL, Li J, Dong HY. Insight into quorum sensing and microbial community of an anammox consortium in response to salt stress: from "Candaditus Brocadia" to "Candaditus Scalindua". The Science of the Total Environment, 2021, 796: 148979. DOI:10.1016/j.scitotenv.2021.148979 |
| [56] | Gu Y, Tian JJ, Zhang Y, Wu R, Li LJ, Zhang BJ, He YF. Dissecting signal molecule AI-2 mediated biofilm formation and environmental tolerance in Lactobacillus plantarum. Journal of Bioscience and Bioengineering, 2021, 131(2): 153-160. DOI:10.1016/j.jbiosc.2020.09.015 |
| [57] | Gunde-Cimerman N, Ramos J, Plemenitas A. Halotolerant and halophilic fungi. Mycological Research, 2009, 113(Pt 11): 1231–1241. |
| [58] | Xu C, Tao S, Li S, Chen L, Zhang W. Adaptive laboratory evolution of cadmium tolerance in Synechocystis sp. PCC 6803. Biotechnology for Biofuels, 2018, 11(1): 205. DOI:10.1186/s13068-018-1205-x |
| [59] | Feng HJ, Zhang XQ, Guo K, Vaiopoulou E, Shen DS, Long YY, Yin J, Wang MZ. Electrical stimulation improves microbial salinity resistance and organofluorine removal in bioelectrochemical systems. Applied and Environmental Microbiology, 2015, 81(11): 3737-3744. DOI:10.1128/AEM.04066-14 |
| [60] | Pathak J, Rajneesh, Singh PR, Häder DP, Sinha RP. UV-induced DNA damage and repair: a cyanobacterial perspective. Plant Gene, 2019, 19: 100194. DOI:10.1016/j.plgene.2019.100194 |
| [61] | Gao YY, Li J, Dong HY, Qiang ZM. Nitrogen removal mechanism of marine anammox bacteria treating nitrogen-laden saline wastewater in response to ultraviolet (UV) irradiation: high UV tolerance and microbial community shift. Bioresource Technology, 2021, 320(Pt A): 124325. |
| [62] | Wang HJ, Liu HW, Yu Q, Fan FH, Liu SH, Feng GH, Zhang PY. A CPD photolyase gene PnPHR1 from Antarctic moss Pohlia nutans is involved in the resistance to UV-B radiation and salinity stress. Plant Physiology and Biochemistry, 2021, 167: 235-244. |
| [63] | Ma H, Yang X, Mao J, Zheng H. The energy efficiency prediction method based on gradient boosting regression tree. 2018 2nd IEEE Conference on Energy Internet and Energy System Integration (EI2), 2018: 1–9. |
| [64] | Sun Y, Guan Y, Zeng D, He K, Wu G. Metagenomics-based interpretation of AHLs-mediated quorum sensing in Anammox biofilm reactors for low-strength wastewater treatment. Chemical Engineering Journal, 2018, 344: 42-52. DOI:10.1016/j.cej.2018.03.047 |
| [65] | Small DP, Hüner NPA, Wan W. Effect of static magnetic fields on the growth, photosynthesis and ultrastructure of Chlorella kessleri microalgae. Bioelectromagnetics, 2012, 33(4): 298-308. DOI:10.1002/bem.20706 |
| [66] | Santos LO, Alegre RM, Garcia-Diego C, Cuellar J. Effects of magnetic fields on biomass and glutathione production by the yeast Saccharomyces cerevisiae. Process Biochemistry, 2010, 45(8): 1362-1367. DOI:10.1016/j.procbio.2010.05.008 |
| [67] | Kovacs PE, Valentine RL, Alvarez PJJ. The effect of static magnetic fields on biological systems: implications for enhanced biodegradation. Critical Reviews in Environmental Science and Technology, 1997, 27(4): 319-382. DOI:10.1080/10643389709388503 |
| [68] | Cardenas-Robles A, Martinez E, Rendon-Alcantar I, Frontana C, Gonzalez-Gutierrez L. Development of an activated carbon-packed microbial bioelectrochemical system for azo dye degradation. Bioresource Technology, 2013, 127: 37-43. DOI:10.1016/j.biortech.2012.09.066 |
| [69] | Zhang JX, Zhang YB, Quan X. Electricity assisted anaerobic treatment of salinity wastewater and its effects on microbial communities. Water Research, 2012, 46(11): 3535-3543. DOI:10.1016/j.watres.2012.03.059 |
| [70] | Hartke A, Frère J, Boutibonnes P, Auffray Y. Differential induction of the chaperonin GroEL and the co-chaperonin GroES by heat, acid, and UV-irradiation in Lactococcus lactis subsp. lactis. Current Microbiology, 1997, 34(1): 23-26. DOI:10.1007/s002849900138 |
| [71] |
Zhang L, Guo LZ, Lu WD. Construction of salt sensitive mutants from Halomonas sp. TTW4 and cloning of the gene involved in cellular osmoregulation. Journal of Qingdao Agricultural University: Natural Science, 2013, 30(3): 204-210.
(in Chinese) 张琳, 郭立忠, 卢伟东. Halomonas sp. TTW4盐敏感突变株的筛选与耐盐基因克隆. 青岛农业大学学报: 自然科学版, 2013, 30(3): 204-210. |
| [72] | Niu C, Geng J, Ren H, Ding L, Xu K, Liang W. The strengthening effect of a static magnetic field on activated sludge activity at low temperature. Bioresource Technology, 2013, 150: 156-162. DOI:10.1016/j.biortech.2013.08.139 |
| [73] | Niu C, Liang W, Ren H, Geng J, Ding L, Xu K. Enhancement of activated sludge activity by 10–50 mT static magnetic field intensity at low temperature. Bioresource Technology, 2014, 159: 48-54. DOI:10.1016/j.biortech.2014.01.131 |
| [74] | Shao Y, Mu G, Song L, Yan S, Tan L. Enhanced biodecolorization performance of azo dyes under high-salt conditions by a marine microbial community exposed to moderate-intensity static magnetic field. Environmental Engineering Science, 2019, 36(2): 186-196. DOI:10.1089/ees.2018.0188 |
| [75] | Wang YM, Xu BW, Ning SX, Shi SN, Tan L. Magnetically stimulated azo dye biodegradation by a newly isolated osmo-tolerant Candida tropicalis A1 and transcriptomic responses. Ecotoxicology and Environmental Safety, 2021, 209: 111791. DOI:10.1016/j.ecoenv.2020.111791 |
 2022, Vol. 62
2022, Vol. 62




