中国科学院微生物研究所,中国微生物学会
文章信息
- 王侧容, 郑春霞, 张漫漫, 吴启凤, 何腾霞. 2023
- WANG Cerong, ZHENG Chunxia, ZHANG Manman, WU Qifeng, HE Tengxia.
- 微生物异化硝酸盐和亚硝酸盐产铵研究进展
- Research progress in ammonium production from microbial dissimilatory reduction of nitrate and nitrite
- 微生物学报, 63(4): 1340-1355
- Acta Microbiologica Sinica, 63(4): 1340-1355
-
文章历史
- 收稿日期:2022-09-06
- 网络出版日期:2022-11-21
氮(nitrogen, N)循环作为地球生物化学的基础循环之一,对内陆和水生生态系统中微生物、藻类、植物的生长发育具有重要作用[1]。而铵态氮(ammonium nitrogen, NH4+)作为氮循环中的重要组成部分,可来源于农业生产、工业废水、生活污水和生物转化等方面[2-3],是植物生长所需的主要无机氮之一[4],被植物优先利用[5]。目前针对NH4+的研究主要集中在土壤、海洋、硝化污泥和人工湿地等环境。NH4+对硝化作用、NO2的排放[6]、土壤中细菌群落的形成、微生物多样性以及相关功能的表达均具有重要影响[7]。据文献报道,氨(ammonia, NH3)经过哈伯-博施(Haber-Bosch)工艺合成后,在酸性物质的作用下,可被有效中和转化为NH4+[8-9],但这种方法不仅价格昂贵、耗费能源[10],且转化效率有限。因此,有必要研究更绿色友好的铵合成替代技术以提升铵的合成效率和减轻环境污染问题。另外,据研究表明,反硝化和氨氧化工艺是生物脱氮中氮损失的主要方式[11]。因此,为了满足氮需求,控制氮素流失和滞留对维持生态系统的氮平衡具有重要意义。基于此,微生物异化硝酸盐和亚硝酸盐(nitrite, NO2−)还原产铵的探索逐渐获得研究者们的青睐,并成为氮回收的研究热点。
据报道,硝酸盐的还原主要包括同化硝酸盐还原(assimilatory nitrate reduction to ammonium, ANRA)和异化硝酸盐还原(dissimilatory nitrate reduction to ammonium, DNRA)[12-13]。ANRA是指经过中间体NO2−将NO3−还原成NH4+,最后NH4+形成生物量氮(如氨基酸或蛋白质)的过程[14-15],而异化硝酸盐还原根据终产物的差异分为两类:(1) 反硝化过程;(2) 异化硝酸盐还原产铵[12-13]。反硝化的产物包括亚硝酸盐、N2、NO、N2O等。而同化硝酸盐还原和异化硝酸盐还原的区别主要在于:(1) ANRA是利用环境中的NO3−合成生物量氮,而异化硝酸盐还原是将NO3−和NO2−还原为N2、N2O和NH4+[16];(2) ANRA是耗能过程,异化硝酸盐还原是产能过程[12];(3) 参与反应过程的酶种类不同。ANRA主要是同化硝酸盐还原酶(nitrate reductase, NAS)和同化亚硝酸盐还原酶,而异化硝酸盐还原包括了周质硝酸盐还原酶(periplasmic nitrate reductase2, NAP)、周质亚硝酸盐还原酶(periplasmic nitrite reductase, NRFA)和一氧化氮还原酶(nitric oxide reductase, NOR)等[15];(4) ANRA产生的NH4+全部用于生物量氮的合成,而DNRA过程产生的NH4+部分可用于生物量氮的合成[17]。与此同时,NO2−和NO3−是微生物硝化过程的中间产物,可造成水体富营养化,而DNRA作为一种氮保存方式,且异化亚硝酸盐还原产铵作为DNRA过程中的关键反应[15],可将NO2−和NO3−还原为更易于生物生长的NH4+[18-20]。研究表明,DNRA有着节能环保、降低温室气体N2O排放量[15]、保留氮肥以及减少废水中NO2−和NO3−污染的优点[21]。
放线菌Streptomyces mediolani EM-B2[22]的DNRA途径可能比其他细菌更短,具有节能、高效、环保、产铵多的优点,因此详细了解微生物的DNRA过程和EM-B2在DNRA中的产铵特点有利于进一步控制氮流失以及避免氮流失引起的环境污染问题。而目前,参与DNRA过程的微生物多数是细菌,对放线菌的DNRA过程研究较为缺乏,通过查阅大量文献,未见关于DNRA发生途径的系统性报道,本文详细地论述了参与DNRA微生物的种类、DNRA的发生途径及其积累的中产物。比较分析了放线菌和其他微生物通过DNRA过程产铵的差异,并讨论了菌株EM-B2进行DNRA的优势,预测了菌株EM-B2在混合氮源中通过DNRA过程产铵的机制,最后总结了后续铵的处理,讨论了回收铵的难点以及有关该研究面临的挑战。本综述可为进一步研究细菌通过DNRA的氮代谢过程积累铵态氮提供方向,并为今后建立放线菌通过DNRA工艺产铵提供理论支持。
1 DNRA微生物的种类纯培养下的DNRA现象早在1938年就已被Woods[23]发现,随着科学技术的发展,许多微生物均被发现具有DNRA的能力,且不同的环境由不同的微生物主导。按照对氧气耐受性的不同,DNRA微生物分为严格厌氧型、兼性厌氧型、微好氧型和好氧型[24]。根据微生物获取能量的方式以及生长底物的差异,DNRA微生物又分为发酵型和呼吸型[25],其中发酵型一般指异养型,是指微生物以有机碳作为电子供体从而获取能量,如葡萄糖通过底物水平磷酸化还原产NH4+[26];而呼吸型一般指自养型,是指微生物以无机碳作为电子供体,如细菌以S2−或Fe2+作为电子供体,将NO3−还原为NH4+,最终S2−变成单质S或SO42−,Fe2+变成Fe3+[27]。据文献报道,硫化物的缺乏会导致自养型DNRA微生物丰度降低,从而形成弱DNRA环境、影响DNRA速率[28],如Lu等[29]表明当S/N增高到一定比例时,DNRA过程产生的NH4+会相应增多,且Li等[28]也表明硫化物可能支持化能自养DNRA微生物的生长并促进DNRA速率,该理论与Brunet等[30]的报道类似。此外,自养型DNRA无需外加碳源,因此在减少有机碳残留的同时,还能去除NO3−、NO2−、硫化物和铁化物等[31]。但与异养型相比,自养型DNRA微生物的生物量产量和同化效率较低且生长缓慢[32]。其次,在目前的多数研究中,参与DNRA的微生物大多都是异养型,如细菌Aeromonas[33]、Lacunisphaera[5]、真菌Calocybegambosa[34]等;自养型的DNRA微生物较少,如Beggiatoa[35]和Sulforospirillum deleyianum[36],且在废水处理中能进行有效DNRA的自养微生物报道更为有限。此外,Zhang等[37]研究发现自养DNRA细菌和异养DNRA细菌可以共存,但其共存的确切机制还需进一步探讨。
现阶段DNRA微生物的分类大多处于呼吸型和发酵型这一层面,对其分类研究较浅,而最新研究表明,微生物群落的物种组成及其氮代谢途径还受到环境变化的影响[38],其中季节性变化引起的温差对其起着决定性作用[39]。如Li等[40]研究中国温带至热带气候梯度沿线河流沉积物中DNRA过程的空间分布时,发现DNRA受到河流气候的强烈影响,且DNRA速率沿温带至热带气候梯度的递增而增加;Rahman等[41]在DNRA细菌活化能的研究分析中发现了存在嗜冷和嗜温的DNRA细菌群落,因此,DNRA微生物的分类逐渐有了温度划分,该理论得到了Wang等[5]的证实,其研究结果表明,DNRA细菌的群落结构主要受到温度的影响,并将DNRA细菌分为低温、中温和高温簇。根据DNRA细菌这一特点,本文对不同种属的DNRA微生物进行了详细论述,如表 1所示。
| Chemical energy type | Strain | Breathing type | T/℃ | Separation point | References |
| Heterotrophic | Vibrio sp. Y1-5 | Aerobic | 25−35 | Surface sediments of Jiaozhou Bay, China | [46] |
| Anaeromyxobacter | Facultative anaerobic | > 25 | Guangzhou Sewage Treatment Plant | [5] | |
| Brocadia | Facultative anaerobic | > 20 | Chongqing Sewage Treatment Plant | [5] | |
| Nitrospira | Facultative anaerobic | ~15 | Beijing Sewage Treatment Plant | [5] | |
| Gethrix | Facultative anaerobic | ~5 | Harbin Sewage Treatment Plant | [5] | |
| Lacunisphaera | Facultative anaerobic | ~15 | Changchun Sewage Treatment Plant | [5] | |
| Pseudomonas putida strain NP5 |
Facultative anaerobic | 10−37 | Sludge obtained from a piggery wastewater treatment system (Xi’an, China) | [47] | |
| Streptomyces mediolani EM-B2 |
Aerobic | 15−40 | A biogas digester in Huaxi District on the southern side of Guiyang City | [48] | |
| Thermosulfurimonas marina SU872T | Anaerobic | 50−79 | A shallow-sea hydrothermal vent in the Pacific Ocean near Kunashir Island | [49] | |
| Chemolithoautotrophic | Thermosulfuriphilus ammonigenes gen ST65T | Anaerobic | 47−75 | A deep-sea hydrothermal vent chimney located on the Eastern Lau Spreading Centre In the south-western Pacific Ocean |
[50] |
| Autotroph | Thermosulfurimonas dismutans | Anaerobic | Thermophilic optimal growth Temperature 74 ℃ |
Deep-sea hydrothermal vents | [51] |
| Autotroph | Dissulfuribacter thermophilus | Anaerobic | Thermophilic optimal growth temperature 61 ℃ |
Deep-sea hydrothermal vents | [51] |
| Mixotrophic | Nautilia profundicola Am-H | Strictly Anaerobic | 30−55 | Deep-sea hydrothermal vents | [52-53] |
| Chemolithoautotrophic | Caminibacter hydrogeniphilus gen.AM1116T | Anaerobic | 50−70 | An East Pacific Rise hydrothermal vent sample | [54] |
综上,在不同温度条件下,DNRA微生物群落具有差异性的这一生理特性,可以在相应的生境中分离出对应功能的DNRA菌,并将其应用于不同温度条件的污泥、废水和土壤中进行无机氮转化,以达到高效回收氮的目的,对于这些DNRA微生物是否广泛分布以及它们对温度变化的适应能力如何,还需进一步挖掘。就目前的研究而言,高温环境更利于DNRA过程的发生,其产生原因是高温条件可以增强有机碳的分解和淋溶,为DNRA提供更多的溶解有机碳和氧化有机碳[42];而低温DNRA微生物在寒冷条件下依然能进行DNRA过程的原因可能是冷休克和能量产生相关基因共同协调的结果,如细菌会产生一些抗冻蛋白[43],又或是细胞中一些高含量的氨基酸和糖发挥作用[44],致使细菌在低温下仍能保持较高的细胞活力[45],但对于低温环境下DNRA贡献率低,以及有关适应寒冷或冷冻应激的分子机制尚不清楚,推测贡献率低的可能原因是低温条件下酶活性受到抑制,或在DNRA发生的过程中能量和生长底物的缺乏限制了DNRA微生物的生长繁殖,从而限制DNRA过程的发生,但这一推测有待进一步验证。另外,目前的多数研究均是针对不同季节的沉积物和温度对DNRA速率的影响,对于DNRA微生物群落对应的温度详细分类、影响DNRA微生物群落分布的其他因素,以及低温簇DNRA微生物的发现研究较少。考虑到土壤、废水、河流等处理系统的多样性和复杂性,如在冬季,中温或高温簇DNRA微生物可能会面临巨大的挑战和限制,因此,未来可以通过基因工程技术改造菌株的基因,以获取更多具有特异性功能的DNRA微生物,发掘更强的DNRA微生物,并加强功能DNRA微生物在各种环境条件中的耐受研究。
2 DNRA的机理及途径 2.1 DNRA的发生机理DNRA过程作为氮循环重要的组成部分之一,对NO2−和NO3−从各种处理系统中还原为NH4+具有重大意义。据报道,参与DNRA过程的酶主要有硝酸还原酶和亚硝酸还原酶。硝酸还原酶可分为呼吸硝酸还原酶(respiratory nitrate reductase, NAR)、NAP和NAS[55];NAP在有氧和无氧条件下均能表达,但在有氧条件下占主导,主要发生在DNRA过程中[56-57];而NAR仅在无氧条件下表达,主要发生在反硝化过程中[56];NAS是位于细胞质中,用于ANRA过程[46]。亚硝酸还原酶有胞质NADH依赖性亚硝酸还原酶(nitrite reductase, NIR)、NRFA和同化亚硝酸还原酶[25, 58]。在DNRA过程中,NO3−/NO2−还原酶有两个组合,即NAP/NRFA和NAR/NIR[26]。NAP/NRFA位于DNRA微生物的周质(细胞膜外),而NAR/NIR存在于细胞质中[26, 59]。DNRA反应的第一步是NO3−到NO2−,其过程是与膜结合的NAR将细胞质膜上的NO3−还原为NO2−并将其释放到细胞质中,NIR再将NO2−还原为NH4+[26];或者是周质上的NAP将NO3−还原为NO2−,再由NRFA将NO2−转化为NH4+。而在ANRA过程中,首先是NO3−被NAS还原为NO2−,然后NO2−被同化亚硝酸酶还原为NH4+[16],最终,在谷氨酸脱氢酶的作用下,NH4+被转化为谷氨酸用于细胞生长,合成生物量氮[60]。
2.2 微生物异化硝酸盐和亚硝酸盐还原产铵的途径据了解,微生物异化还原产铵的途径多种多样,且大多数报道的DNRA途径为NO3−→NO2−→ NH4+,如在1980年,Caskey等[61]就已经在菌株Clostridium KDHS2的硝酸盐还原实验研究中,发现了唯一中间体NO2−的产生和还原产物NH4+的生成,即DNRA途径为NO3−→NO2−→ NH4+;Zhou等[62]也在真菌Fusarium oxysporum MT-811的硝酸盐代谢机制研究中,通过实验分析得出该菌的DNRA途径为NO3−→NO2−→NH4+;但在Ding等[63]对砂岩表面DNRA驱动NH4+的内部循环机制研究中,通过宏基因组和15N同位素分析,发现NO3−可直接还原为NH4+的短程路径,该途径的发现得到了Lam等[64]的证实。除此以外,He等[65]以NO2−作为唯一氮源时,因实验过程中并未检测到NO3−和NH2OH,且有NH4+的积累,因此,根据实验结果推测菌株Arthrobacter arilaitensis Y-10可将NO2−转化为NH4+,即NO2−→NH4+。随后,He等[66]在探究不同Mn(Ⅱ)浓度下菌株Pseudomonas taiwanensis J488的亚硝酸盐型反硝化脱氮能力研究中,发现以NO2−为唯一氮源且Mn(II)浓度逐渐从0 mg/L增加到30 mg/L的过程中,NH4+浓度也从0 mg/L积累到了3.9 mg/L,反硝化过程中NH4+产生的原因并不是源于裂解死亡的细胞。由于微生物种类的差异,一些细菌可将NO2−直接还原为NH4+,因此,推测菌株J488积累铵的途径可能与异化亚硝酸盐还原产生铵有关,且途径为NO2−→NH4+,该途径在Song等[67]的文章中也得到了进一步的论证。目前来看,NO3−→NH4+和NO2−→NH4+的直接还原途径相较于NO3−→NO2−→NH4+具有耗能小、副产物少、耗时短的优点,但NO3−和NO2−直接还原为NH4+的详细还原机制和还原效率还未揭示,需进一步探索。另外,Jin等[68]通过研究证实了约38.75%的羟胺(0.104 mmol/L)可直接催化生成1.85 mg/L的NH4+ (0.103 mmol/L),且反应过程中未发现NO3−或NO2−的积累,但该文未报道NH2OH通过微生物进行DNRA过程的完整途径;而Hanson等[69]通过研究发现,在菌株Nautilia profundicola AmH中,NO3−被还原为NO2−后,在依赖醌的羟胺脱氢酶催化作用下,NO2−可转化为NH2OH,随后NH2OH被转运至细胞质中,并通过羟胺还原酶(也称为杂合簇蛋白(Har/Hcp))还原为NH4+,该途径(NO3−→NO2−→NH2OH→NH4+)的发现在Campbell[52]和Kostera等[70]的研究中也得到了证明。与Hanson、Campbell和Kostera等人的研究不同,Burlat等[71]报道的羟胺还原酶种类和DNRA发生途径不一样,该研究表明NH2OH还原为NH4+由酶NRFA催化,并且还有研究表明NH2OH可由羟胺还原酶(HAO)催化还原为NH4+,其途径为NO3−→NO2−→NO→NH2OH→ NH4+[72-74],其次,聂铭和Kostera等[75]人还发现εHAO可催化NO转化为NH4+,其途径为NO3−→NO2−→NO→NH4+。然而,NO和NH2OH还原为NH4+的效率低[74],且NO还原为NH4+的过程还受到O2的影响,因此,在该途径中提高产铵效率并减少温室气体的排放还面临较大挑战。另外,NH2OH→NH4+、NO→NH2OH→NH4+和NO→NH4+的途径研究较少,推测可能与DNRA微生物的种类、酶和氮代谢相关的基因差异、酶的还原能力与亲和力有关,因为并不是任何具有DNRA能力的微生物都有NRFA基因的同工酶[76]。
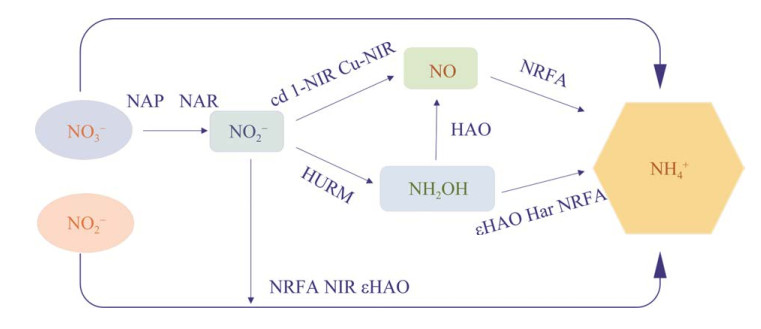
综上,NO→NH4+、NH2OH→NH4+和NO→ NH2OH→NH4+途径的发现不仅完善了DNRA过程,也为人类研究生态系统中NH2OH的减少以及氮循环的发生途径提供新思路。未来可通过以下几种方式提高NH4+产量:(1) 对εHAO与Har酶的基因进行调控从而使其大量表达;(2) 通过NO3−和NO2−的有效利用,提高NH4+产量;(3) 挖掘更多新的DNRA短程还原途径,提高NH4+产量。本文首次对DNRA的发生机理和途径进行了总结和分析,如图 1所示。目前,DNRA的发生过程共有6个途径:(1) NO3−→NO2−→NH4+;(2) NO3−→NH4+;(3) NO2−→NH4+;(4) NO3−→ NO2−→NO→NH4+;(5) NO3−→NO2−→NH2OH→ NH4+;(6) NO3−→NO2−→NO→NH2OH→NH4+。
|
| 图 1 微生物异化硝酸盐和亚硝酸盐还原产铵的途径 Figure 1 The microbial pathway of ammonium production by dissimilatory nitrate and nitrite reduction. |
综上,DNRA途径多种多样,随着DNRA研究的不断深入,一些新的DNRA途径也会逐渐被发现,对于这些新的途径是以何种代谢机制产铵以及涉及哪些基因参与的仍需进一步求证。目前国内外对硝酸盐或亚硝酸异化还原产铵的研究还不够深入,在DNRA的发生途径、相关的氮代谢还原酶和功能基因表达情况等方面的研究报道较浅,未来可以从分子水平上加深对DNRA微生物产铵的研究,并加强DNRA工艺在各种废水与污泥中的工程运用。
3 不同氮源底物对异化还原产铵的影响研究随着DNRA研究的持续推进,关于不同氮源对DNRA产物影响的研究也越来越多,并取得了一定的成果,目前主要集中在单一氮源和混合氮源对DNRA速率以及产物积累的影响上,大多数DNRA产物的种类和积累量会因氮底物(NH4+、NO2−和NO3−)性质的差异而发生不同反应[77]。如在相同浓度的NO3−或NO2−为唯一氮源的代谢机制研究中,Su等[78]发现在0 g/L的零价铁和菌株Desulfovibrio sp.CMX的存在下,以NO3−和NO2−分别作为唯一氮源时,5 mmol/L的NO3−会产生3.86 mmol/L的NH4+,而5 mmol/L的NO2−会产生更多的NH4+ (4.52 mmol/L),该结果与He等[66]的研究类似,其研究结果表明当菌株Arthrobacter arilaitensis Y-10以101.04 mg/L的NO3−为氮源时,在72 h后积累了43.66 mg/L的NO2−,无NH4+积累,而以100.03 mg/L的NO2−为氮源时,在72 h后却会产生2.15 mg/L的NH4+。
此外,同一氮源不同浓度或多种底物共存于同一纯培养物反应系统时,氮平衡的构建会变得更加复杂[77],菌株的代谢途径、系统中氮代谢的产物种类以及积累量会出现较大差异。如Xie等[79]在不同NO2−浓度下研究菌株Pseudomonas mendocina X49的反硝化能力,结果发现当NO2−初始浓度为100 mg/L时,产生的NH4+最多为6.76 mg/L,且没有NO3−的积累,此发现与Sun等[80]的结果类似,其表明在浓度分别为1、5、10 mmol/L的亚硝酸盐培养基中,菌株Bacillus paralicheniformis LMG6934在培养72 h后,通过异化亚硝酸还原分别产生了0.99、4.35、8.53 mmol/L的NH4+,以上研究表明NO2−浓度越高,产生的NH4+浓度也会随之增加。
另外,对于多种氮底物共存于同一个培养物系统时,He等[22]发现在同时硝化与反硝化(SND)过程中,当混合氮源为53.92 mg/L的NH4+与50.59 mg/L的NO3−时,接种24 h后,NH4+几乎被消耗掉,去除率为98.96%,NO3−仅去除了14.29%,表明菌株EM-B2优先利用NH4+再利用NO3−,继续培养48 h后,NO3−的去除率达到了87.15%,NH4+浓度趋于稳定,又培养至100 h后,NO3−与NH4+浓度均趋于稳定,无明显变化,这与Li等[46]报道一致,值得注意的是,在混合氮源为50 mg/L NO2−与50 mg/L NO3−的培养基中,NO2−与NO3−的去除率在48 h内分别达到了100%和83.57%,之后,在20−96 h内,NH4+持续积累并达到了26.03 mg/L,而在混合氮源为NO2−与NH4+的培养中也出现了类似现象,以50 mg/L NO2−与50 mg/L NH4+作为混合氮源时,在接种0−48 h内,NH4+与NO2−二者同时被去除,去除速率几乎一致,培养48 h后,NO2−的去除率高达100%,NH4+剩余4.30 mg/L,而在48−100 h的培养阶段,NH4+的含量逐渐增加,直至31.61 mg/L。因此,推测在48 h前,NH4+已经被耗尽且在反应系统中重新开始生成,故在48 h时检测到4.30 mg/L的NH4+。因此,推测NH4+的积累均与底物NO2−有关,且NO2−在异化还原产铵中扮演着重要的角色,这可能与菌株对底物作用的酶有关[81],如Ma等[82]研究表明NH4+会作为类似于NO2−的诱导剂,可促进NO2−的同化还原。Eisenmann等[36]研究也发现菌株Sulfurospirillum deleyianum形成NH4+的亚硝酸还原酶是一种诱导型酶,当细胞用硝酸盐、亚硝酸盐或元素硫培养时均会表达,但在用富马酸盐培养时会被抑制。因此,推测在NO2−存在条件下,其他氮源对亚硝酸盐还原酶起诱导作用,如Jin等[68]发现,与单一氮源相比,亚硝酸还原酶(NIRBD)仅在混合氮源下会使菌株发生大量的同化作用,结合菌株Streptomyces mediolani EM-B2,在NH4+与NO3−的混合培养中,因为菌株没有NO2−的大量积累,所以无法加强同化作用,但在NO2−与NO3−,NO2−与NH4+的混合氮源中,因为有高浓度的NO2−存在,所以大量的无机氮可异化还原为NH4+,因此推测存在于菌株EM-B2的亚硝酸还原酶也是一种诱导型还原酶,但该推论还需后续验证。此外,也有研究表明,在不同氮浓度的培养基中,羟胺还原酶基因的表达水平不一样[69],如Jin等[68]发现在低浓度铵(< 100 mg/L)的存在下,HAR表达水平不受影响,但当初始NH4+浓度为300 mg/L与800 mg/L时,HAR的表达会急剧增加。基于此,推测菌株EM-B2的亚硝酸还原酶可能也受到初始NO2−浓度的影响,从而启动独特的异化还原产铵机制,也就有了菌株EM-B2在NO2−与NO3−,NO2−与NH4+的混合氮源中,分别积累26.03 mg/L和31.61 mg/L NH4+的现象,但这一推测需后续验证。综上,菌株EM-B2似乎为DNRA提供了新的替代途径,与一般的DNRA微生物明显不同,且据我们所知,菌株EM-B2是在混合氮源培养下的HN-AD中NH4+积累较多的首个报告,其优异的产铵性能表明菌株EM-B2中存在独特的氮转化途径或功能酶,对氮的回收具有重大意义,因此,需重视菌株EM-B2异化硝酸盐和亚硝酸盐还原产铵机制的研究。
现阶段国内外对放线菌治理氮污染废水的研究主要集中在放线菌的生长、氮转化途径及其影响因素方面,如温度、无机氮浓度和化学需氧量(chemical oxygen demand, COD)等,很少有研究同时去探讨放线菌中的HN-AD和DNRA两种过程以及与氮代谢相关的基因和酶,并且只有少数放线菌被检测出与氮代谢相关的酶类,因此,未来对放线菌的分子机制应给予重视。其次,HN-AD研究报道均以细菌为主,而对于放线菌在异化还原产铵方面的研究、DNRA过程的转化机制和相关功能基因尚不清楚,因此,有必要进一步筛选出更多产NH4+能力强的放线菌,并研究这些菌株的氮代谢途径、相关基因表达以及两者之间的关系。总体而言,菌株EM-B2在混合氮源中产NH4+的能力远远高于其他的菌株,在处理含无机氮废水和节约氮资源方面具有以下优点:(1) 在单一或混合氮源中都可以高效地去除高浓度的无机氮(NH4+、NO2−、NO3−、NH2OH),具有高转化率与中间产物积累较少的优点;(2) 能抵抗高浓度的NO2−、高浓度的溶解氧且具有耐碱性,在7.6 mg/L的高溶氧条件下仍有活性,比菌株Streptomyces sp. XD-11-6-2[83]、Desulfuromusa和Denitrovibrio[84]更能抵抗高浓度的溶解氧;且当pH为9.0时,对NH4+的去除率仍高达99.61%,比菌株Pseudomonas putida Y-9[85]更耐碱;(3) 耐受高低温环境(15−40 ℃),对气候条件的依赖性小;(4) 在NH4+与NO2−,NO2−与NO3−的混合氮源中,具备较强的产NH4+能力。本课题组会持续推进菌株EM-B2参与的DNRA的研究,如对菌株EM-B2进行转录组测序和全基因组测序,从分子水平和代谢水平准确全面地分析参与DNRA的功能基因及其相应丰度。此外,DNRA途径会因为微生物种类的不同存在差异性,而菌株EM-B2是从牛粪发酵沼气池中分离获得的放线菌,该菌株中可能存在一些未被发现的关于DNRA的基因、酶或新的产铵代谢途径,这对于扩大放线菌在氮污染废水的生物修复领域和补全目前放线菌在氮代谢方面的缺失具有重大意义。
4 异化还原后铵的去向密切关注废水中NH4+的去向对氮的高效回收是至关重要的。NO3−或NO2−异化还原为NH4+后,NH4+主要有以下几个去向:(1) 满足细胞合成的需要,转化为生物量氮[15, 17];(2) 还原后的NH4+又重新返回系统,进行氨氧化反应[58];(3) 生成的NH4+转化为NO3−从而增强反硝化作用[86];(4) 通过调节废水的pH使NH4+挥发为NH3,之后再通过空气汽提法进行回收[87];(5) NH4+挥发后逃逸到大气中[88];(6) 由初级生产者重新使用[84],如藻类和光合细菌[87];(7) 滞留在系统中的NH4+通过各种技术方法回收。如鸟粪石结晶[89]、天然沸石[90]、空气汽提[91]、离子交换吸附、膜分离技术、活性污泥,光合细菌等[87]。但在日常的废水中,NH4+经过稀释后含量较少,使用物理化学法需要消耗大量能源、且成本高昂、操作严格,因此在低氮废水中不可行,物理化学法仅仅适用于高浓度(> 1 000 mg/L)的氮废水处理[92],而在 < 50 mg/L的低浓度氮废水中,可以通过物理、化学或生物相结合的方法来提取NH4+,如三层聚乙烯醇凝胶[93]。目前研究表明汽提法因操作简便、污染小、能源需求和成本相对较低的优点被证明是最适用的提铵技术[94]。随着技术的更新,微生物燃料电池(MFC)、微生物电化学电池(MEC)和微生物脱盐电池(MDC)等方法也逐渐显现并应用[10, 95],但这些方法仍处于研发阶段[96],并且任何的NH4+回收策略都有优缺点,且均达不到100%的NH4+回收,因此在土壤、废水、污泥等处理系统中通过DNRA提高NH4+的浓度,是提高NH4+回收的有效方法,而提高处理系统中NH4+的浓度需高产NH4+的微生物参与转化,如Streptomyces mediolani EM-B2。另外,全球城市污水处理厂不可避免地会产生大量的废弃活性污泥(WAS)[92],而氮的回收都主要应用于废水,因此,将具有DNRA功能的微生物应用于废弃活性污泥中的氮回收具有经济高效与可持续性的优点,基于以上论述,未来有必要寻找一种从污泥中高效回收氮的方法,将回收的氮用于补充肥料,减轻环境污染和减少氮肥生产所需的能源成本问题。
5 结论与展望目前关于硝酸盐或亚硝酸盐异化还原产铵的研究主要集中在微生物的生长条件,如C/N、温度、pH等方面,对该类微生物群落分布的影响因素、各类微生物的异化还原产铵机制、代谢途径和放线菌的异化还原产铵等方面的研究还比较匮乏,探明硝酸盐或亚硝酸盐异化还原产铵机制有助于氮的高效回收和减少氮的损失,对生态系统中的氮循环具有重大意义。本文系统综述了DNRA微生物的种类、DNRA的机理和发生途径,比较了不同氮源底物对异化还原产铵的影响,最后讨论了铵的回收处理,发现目前还存在许多尚未解决的问题,因此,后续可从以下几个方面进行探究:
(1) 进一步剖析细菌的DNRA机制,解析产铵量与功能基因和酶的相关性,并挖掘和证实更多新的异化还原产铵途径。如探究羟胺还原酶基因在DNRA微生物中的生理作用,羟胺还原酶和产铵量之间的相关性等。
(2) 深度分析DNRA代谢过程的相关基因是如何同时响应或参与SND途径的。如DNRA与SND过程的耦合研究,有助于明确氮素转化规律以及DNRA与SND的竞争机制。
(3) 增加放线菌利用多种氮源进行异化还原产铵的研究。如菌株EM-B2在NO2—与NH4+或NO2−与NO3−混合氮源中的高产铵量,打破了以往人们对DNRA菌群以细菌为主的认知,其异化还原产铵的机理值得深入研究。
(4) 通过分子生物技术方法,筛选和改造出更多的DNRA微生物,提高菌株的产铵能力。如利用基因编辑技术对DNRA微生物的相关还原酶基因进行敲除或克隆,使还原产铵的相关基因大量表达。
(5) 优化回收铵的方法。如可通过技术间的结合开发出更绿色环保、低成本、低能耗和应用范围更广的氮回收技术,从而达到氮回收效率最大化以及回收技术工业化的目的。
| [1] | FRANCIS CA, BEMAN JM, KUYPERS MMM. New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. The ISME Journal, 2007, 1(1): 19-27. DOI:10.1038/ismej.2007.8 |
| [2] | GENG J. Effects of N deposition on the quality and quantity of soil organic matter in a boreal forest: contrasting roles of ammonium and nitrate. CATENA, 2021, 198: 104996. DOI:10.1016/j.catena.2020.104996 |
| [3] | LIANG Y, MA R, NGHIEM A, XU J, TANG LS, WEI WH, PROMMER H, GAN YQ. Sources of ammonium enriched in groundwater in the central Yangtze River Basin: anthropogenic or geogenic?. Environmental Pollution, 2022, 306: 119463. DOI:10.1016/j.envpol.2022.119463 |
| [4] | ZHANG HQ, ZHAO XQ, SHI Y, LIANG YT, SHEN RF. Changes in soil bacterial communities with increasing distance from maize roots affected by ammonium and nitrate additions. Geoderma, 2021, 398: 115102. DOI:10.1016/j.geoderma.2021.115102 |
| [5] | WANG SY, LIU CL, WANG XX, YUAN DD, ZHU GB. Dissimilatory nitrate reduction to ammonium (DNRA) in traditional municipal wastewater treatment plants in China: widespread but low contribution. Water Research, 2020, 179: 115877. DOI:10.1016/j.watres.2020.115877 |
| [6] | WANG J, TU XS, ZHANG HM, CUI JY, NI K, CHEN JL, CHENG Y, ZHANG JB, CHANG SX. Effects of ammonium-based nitrogen addition on soil nitrification and nitrogen gas emissions depend on fertilizer-induced changes in pH in a tea plantation soil. Science of the Total Environment, 2020, 747: 141340. DOI:10.1016/j.scitotenv.2020.141340 |
| [7] | WANG RQ, ZHANG ZH, LV FJ, LIN HX, WEI LG, XIAO YP. Optimizing the bacterial community structure and function in rhizosphere soil of sesame continuous cropping by the appropriate nitrate ammonium ratio. Rhizosphere, 2022, 23: 100550. DOI:10.1016/j.rhisph.2022.100550 |
| [8] | WANG Y, MEYER TJ. A route to renewable energy triggered by the Haber-Bosch process. Chem, 2019, 5(3): 496-497. DOI:10.1016/j.chempr.2019.02.021 |
| [9] | HERMANN A. Haber und Bosch: brot aus luft-Die ammoniaksynthese. Physik Journal, 1965, 21(4): 168-171. DOI:10.1002/phbl.19650210403 |
| [10] | YE YY, NGO HH, GUO WS, LIU YW, CHANG SW, NGUYEN DD, LIANG H, WANG J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresource Technology, 2018, 268: 749-758. DOI:10.1016/j.biortech.2018.07.111 |
| [11] | THAMDRUP B. New pathways and processes in the global nitrogen cycle. Annual Review of Ecology, Evolution, and Systematics, 2012, 43: 407-428. DOI:10.1146/annurev-ecolsys-102710-145048 |
| [12] |
YIN SX, SHEN QR. Physiological and biochemical characteristics of nitrate reducers in anaerobic soils. Acta Pedologica Sinica, 2003, 40(4): 624-630.
(in Chinese) 殷士学, 沈其荣. 缺氧土壤中硝态氮还原菌的生理生化特征. 土壤学报, 2003, 40(4): 624-630. DOI:10.3321/j.issn:0564-3929.2003.04.021 |
| [13] |
QU JQ, LIU Q, ZHANG QJ, YANG HC, ZHAO M, ZHU H. Regulation of bacterial nitrogen cycle of aquatic plants rhizosphere in the ecological floating beds system for tailwater treatment. Journal of Dalian Ocean University, 2022: 1-14.
(in Chinese) 曲疆奇, 刘青, 张清靖, 杨浩辰, 赵萌, 朱华. 养殖尾水浮床处理系统水生植物根际细菌氮循环作用机制. 大连海洋大学学报, 2022: 1-14. |
| [14] | HUANG XJ, WEISENER CG, NI JP, HE BH, XIE DT, LI ZL. Nitrate assimilation, dissimilatory nitrate reduction to ammonium, and denitrification coexist in Pseudomonas putida Y-9 under aerobic conditions. Bioresource Technology, 2020, 312: 123597. DOI:10.1016/j.biortech.2020.123597 |
| [15] | KUYPERS MMM, MARCHANT HK, KARTAL B. The microbial nitrogen-cycling network. Nature Reviews Microbiology, 2018, 16(5): 263-276. DOI:10.1038/nrmicro.2018.9 |
| [16] |
CHEN LM. Study on the quantity, physiological groups and reduction characteristics of denitrifying bacteria and nitrate reducing bacteria in flooded soil[D]. Yangzhou: Master's Thesis of Yangzhou University, 2001 (in Chinese). 陈丽敏. 淹水土壤中反硝化菌和硝酸还原菌数量、生理类群及其还原特点的研究[D]. 扬州: 扬州大学硕士学位论文, 2001. |
| [17] |
YANG S, WU SJ, CAI YJ, ZHOU WZ, ZHU TB, WANG Y, HUANG P. The synergetic and competitive mechanism andthe dominant factors of dissimilatory nitrate reduction processes: a review. Acta Ecologica Sinica, 2016, 36(5): 1224-1232.
(in Chinese) 杨杉, 吴胜军, 蔡延江, 周文佐, 朱同彬, 王雨, 黄平. 硝态氮异化还原机制及其主导因素研究进展. 生态学报, 2016, 36(5): 1224-1232. |
| [18] | BOEYKENS SP, PIOL MN, SAMUDIO LEGAL L, SARALEGUI AB, VÁZQUEZ C. Eutrophication decrease: phosphate adsorption processes in presence of nitrates. Journal of Environmental Management, 2017, 203: 888-895. DOI:10.1016/j.jenvman.2017.05.026 |
| [19] | KAMEL RM, SHAHAT A, HEGAZY WH, KHODIER EM, AWUAL MR. Efficient toxic nitrite monitoring and removal from aqueous media with ligand based conjugate materials. Journal of Molecular Liquids, 2019, 285: 20-26. DOI:10.1016/j.molliq.2019.04.060 |
| [20] | TIEDJE JM. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Environmental Microbiology of Anaerobes, 1988(April): 179-244. |
| [21] | WANG J, FENG T, CHEN JX, RAMALINGAM V, LI ZX, KABTAMU DM, HE JH, FANG XS. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes. Nano Energy, 2021, 86: 106088. DOI:10.1016/j.nanoen.2021.106088 |
| [22] | HE TX, ZHANG MM, DING CY, WU QF, CHEN MP, MOU SL, CHENG DJ, DUAN SJ, WANG Y. New insight into the nitrogen removal capacity and mechanism of Streptomyces mediolani EM-B2. Bioresource Technology, 2022, 348: 126819. DOI:10.1016/j.biortech.2022.126819 |
| [23] | WOODS DD. The reduction of nitrate to ammonia by Clostridium welchii. The Biochemical Journal, 1938, 32(11): 2000-2012. DOI:10.1042/bj0322000 |
| [24] |
WEI ZM. Preliminary research on dissimilatory nitrate reduction to ammonium in microaerobic condition[D]. Guangzhou: Master's Thesis of South China University of Technology, 2012 (in Chinese). 韦宗敏. 微好氧环境中硝酸盐异化还原成铵的影响研究[D]. 广州: 华南理工大学硕士学位论文, 2012. |
| [25] | MOHAN SB, SCHMID M, JETTEN M, COLE J. Detection and widespread distribution of the nrfA gene encoding nitrite reduction to ammonia, a short circuit in the biological nitrogen cycle that competes with denitrification. FEMS Microbiology Ecology, 2004, 49(3): 433-443. DOI:10.1016/j.femsec.2004.04.012 |
| [26] | PANDEY CB, KUMAR U, KAVIRAJ M, MINICK KJ, MISHRA AK, SINGH JS. DNRA: A short-circuit in biological N-cycling to conserve nitrogen in terrestrial ecosystems. Science of the Total Environment, 2020, 738: 139710. DOI:10.1016/j.scitotenv.2020.139710 |
| [27] | YUAN HZ, JIA BC, ZENG QF, ZHOU YW, WU J, WANG HX, FANG H, CAI YW, LI Q. Dissimilatory nitrate reduction to ammonium (DNRA) potentially facilitates the accumulation of phosphorus in lake water from sediment. Chemosphere, 2022, 303(Pt 1): 134664. |
| [28] | LI XW, BAI D, DENG QH, CAO XY, ZHOU YY, SONG CL. DNRA was limited by sulfide and nrfA abundance in sediments of Xiamen Bay where heterotrophic sulfide-producing genus (Pelobacter) prevailed among DNRA bacteria. Journal of Soils and Sediments, 2021, 21(10): 3493-3504. DOI:10.1007/s11368-021-03013-x |
| [29] |
LU ZL, LI XL, GOU WJ, ZHANG LP. Effect of s/n on process of autotrophic dissimilatory nitrate reduction to ammonium. Environmental Engineering, 2019, 37(12): 17-21.
(in Chinese) 芦昭霖, 李晓玲, 苟文均, 张莉平. S/N对自养硝酸盐异化还原成铵过程的影响. 环境工程, 2019, 37(12): 17-21. |
| [30] | BRUNET RC, GARCIA-GIL LJ. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiology Ecology, 1996, 21(2): 131-138. DOI:10.1111/j.1574-6941.1996.tb00340.x |
| [31] | KLEEREBEZEM R, MENDEZ R. Autotrophic denitrification for combined hydrogen sulfide removal from biogas and post-denitrification. Water Science and Technology: a Journal of the International Association on Water Pollution Research, 2002, 45(10): 349-356. DOI:10.2166/wst.2002.0368 |
| [32] | REZVANI F, SARRAFZADEH MH, EBRAHIMI S, OH HM. Nitrate removal from drinking water with a focus on biological methods: a review. Environmental Science and Pollution Research, 2019, 26(2): 1124-1141. DOI:10.1007/s11356-017-9185-0 |
| [33] | ZHAO YY, BU CN, YANG HL, QIAO ZM, DING SW, NI SQ. Survey of dissimilatory nitrate reduction to ammonium microbial community at national wetland of Shanghai, China. Chemosphere, 2020, 250: 126195. DOI:10.1016/j.chemosphere.2020.126195 |
| [34] | INGELÖG T, NOHRSTEDT HÖ. Ammonia formation and soil pH increase caused by decomposing fruitbodies of macrofungi. Oecologia, 1993, 93(3): 449-451. DOI:10.1007/BF00317891 |
| [35] | TESKE A, SOGIN ML, NIELSEN LP, JANNASCH HW. Phylogenetic relationships of a large marine Beggiatoa. Systematic and Applied Microbiology, 1999, 22(1): 39-44. DOI:10.1016/S0723-2020(99)80026-5 |
| [36] | EISENMANN E, BEUERLE J, SULGER K, KRONECK PMH, SCHUMACHER W. Lithotrophic growth of Sulfurospirillum deleyianum with sulfide as electron donor coupled to respiratory reduction of nitrate to ammonia. Archives of Microbiology, 1995, 164(3): 180-185. DOI:10.1007/BF02529969 |
| [37] | ZHANG T, ZHUANG XM, AHMAD S, LEE T, CAO CB, NI SQ. Investigation of dissimilatory nitrate reduction to ammonium (DNRA) in urban river network along the Huangpu River, China: rates, abundances, and microbial communities. Environmental Science and Pollution Research, 2022, 29(16): 23823-23833. DOI:10.1007/s11356-021-17475-6 |
| [38] | WANG S, ZHI LL, SHAN W, LU H, XU Q, LI J. Correlation of extracellular polymeric substances and microbial community structure in denitrification biofilm exposed to adverse conditions. Microbial Biotechnology, 2020, 13(6): 1889-1903. DOI:10.1111/1751-7915.13633 |
| [39] | ROBERTS KL, KESSLER AJ, GRACE MR, COOK PLM. Increased rates of dissimilatory nitrate reduction to ammonium (DNRA) under oxic conditions in a periodically hypoxic estuary. Geochimica et Cosmochimica Acta, 2014, 133: 313-324. DOI:10.1016/j.gca.2014.02.042 |
| [40] | LI XF, SARDANS J, HOU LJ, GAO DZ, LIU M, PEÑUELAS J. Dissimilatory nitrate/nitrite reduction processes in river sediments across climatic gradient: influences of biogeochemical controls and climatic temperature regime. Journal of Geophysical Research: Biogeosciences, 2019, 124(7): 2305-2320. DOI:10.1029/2019JG005045 |
| [41] | RAHMAN M, GRACE MR, ROBERTS KL, KESSLER AJ, COOK PLM. Effect of temperature and drying-rewetting of sediments on the partitioning between denitrification and DNRA in constructed urban stormwater wetlands. Ecological Engineering, 2019, 140: 105586. DOI:10.1016/j.ecoleng.2019.105586 |
| [42] | HARDISON AK, ALGAR CK, GIBLIN AE, RICH JJ. Influence of organic carbon and nitrate loading on partitioning between dissimilatory nitrate reduction to ammonium (DNRA) and N2 production. Geochimica et Cosmochimica Acta, 2015, 164: 146-160. DOI:10.1016/j.gca.2015.04.049 |
| [43] | DAVIES PL, BAARDSNES J, KUIPER MJ, WALKER VK. Structure and function of antifreeze proteins. Philosophical Transactions of the Royal Society B: Biological Sciences, 2002, 357(1423): 927-935. DOI:10.1098/rstb.2002.1081 |
| [44] | WONG CMVL, BOO SY, VOO CLY, ZAINUDDIN N, NAJIMUDIN N. A comparative transcriptomic analysis provides insights into the cold-adaptation mechanisms of a psychrophilic yeast, Glaciozyma antarctica PI12. Polar Biology, 2019, 42(3): 541-553. DOI:10.1007/s00300-018-02443-7 |
| [45] | HU H, WISNIEWSKI ME, ABDELFATTAH A, ZHENG XD. Biocontrol activity of a cold-adapted yeast from Tibet against gray mold in cherry tomato and its action mechanism. Extremophiles, 2017, 21(4): 789-803. DOI:10.1007/s00792-017-0943-1 |
| [46] | LI YT, WANG YR, FU L, GAO YZ, ZHAO HX, ZHOU WZ. Aerobic-heterotrophic nitrogen removal through nitrate reduction and ammonium assimilation by marine bacterium Vibrio sp. Y1-5. Bioresource Technology, 2017, 230: 103-111. DOI:10.1016/j.biortech.2017.01.049 |
| [47] | YANG L, WANG XH, CUI S, REN YX, YU J, CHEN N, XIAO Q, GUO LK, WANG RH. Simultaneous removal of nitrogen and phosphorous by heterotrophic nitrification-aerobic denitrification of a metal resistant bacterium Pseudomonas putida strain NP5. Bioresource Technology, 2019, 285: 121360. DOI:10.1016/j.biortech.2019.121360 |
| [48] | HE TX, WU QF, DING CY, CHEN MP, ZHANG MM. Hydroxylamine and nitrite are removed effectively by Streptomyces mediolani strain EM-B2. Ecotoxicology and Environmental Safety, 2021, 224: 112693. DOI:10.1016/j.ecoenv.2021.112693 |
| [49] | ALLIOUX M, JEBBAR M, SLOBODKINA G, SLOBODKIN A, MOALIC Y, FROLOVA A, SHAO ZZ, ALAIN K. Complete genome sequence of Thermosulfurimonas marina SU872T, an anaerobic thermophilic chemolithoautotrophic bacterium isolated from a shallow marine hydrothermal vent. Marine Genomics, 2021, 55: 100800. DOI:10.1016/j.margen.2020.100800 |
| [50] | SLOBODKINA GB, REYSENBACH AL, KOLGANOVA TV, NOVIKOV AA, BONCH-OSMOLOVSKAYA EA, SLOBODKIN AI. Thermosulfuriphilus ammonigenes gen. nov., sp. nov., a thermophilic, chemolithoautotrophic bacterium capable of respiratory ammonification of nitrate with elemental sulfur. International Journal of Systematic and Evolutionary Microbiology, 2017, 67(9): 3474-3479. DOI:10.1099/ijsem.0.002142 |
| [51] | SLOBODKINA GB, MARDANOV AV, RAVIN NV, FROLOVA AA, CHERNYH NA, BONCH-OSMOLOVSKAYA EA, SLOBODKIN AI. Respiratory ammonification of nitrate coupled to anaerobic oxidation of elemental sulfur in deep-sea autotrophic thermophilic bacteria. Frontiers in Microbiology, 2017, 8: 87. |
| [52] | CAMPBELL BJ, SMITH JL, HANSON TE, KLOTZ MG, STEIN LY, LEE CK, WU DY, ROBINSON JM, KHOURI HM, EISEN JA, CARY SC. Adaptations to submarine hydrothermal environments exemplified by the genome of Nautilia profundicola. PLoS Genetics, 2009, 5(2): e1000362. DOI:10.1371/journal.pgen.1000362 |
| [53] | SMITH JL, CAMPBELL BJ, HANSON TE, ZHANG CL, CARY SC. Nautilia profundicola sp. nov., a thermophilic, sulfur-reducing Epsilonproteobacterium from deep-sea hydrothermal vents. International Journal of Systematic and Evolutionary Microbiology, 2008, 58(Pt 7): 1598-1602. |
| [54] | ALAIN K, QUERELLOU J, LESONGEUR F, PIGNET P, CRASSOUS P, RAGUÉNÈS G, CUEFF V, CAMBON-BONAVITA MA. Caminibacter hydrogeniphilus Gen. nov., sp. nov., a novel thermophilic, hydrogen-oxidizing bacterium isolated from an East Pacific Rise hydrothermal vent. International Journal of Systematic and Evolutionary Microbiology, 2002, 52(Pt 4): 1317-1323. |
| [55] | GONZÁLEZ PJ, CORREIA C, MOURA I, BRONDINO CD, MOURA JJG. Bacterial nitrate reductases: molecular and biological aspects of nitrate reduction. Journal of Inorganic Biochemistry, 2006, 100(5/6): 1015-1023. |
| [56] | YANG JX, FENG L, PI SS, CUI D, MA F, ZHAO HP, LI A. A critical review of aerobic denitrification: insights into the intracellular electron transfer. Science of the Total Environment, 2020, 731: 139080. DOI:10.1016/j.scitotenv.2020.139080 |
| [57] | YANG T, XIN Y, ZHANG L, GU ZH, LI YR, DING ZY, SHI GY. Characterization on the aerobic denitrification process of Bacillus strains. Biomass and Bioenergy, 2020, 140: 105677. DOI:10.1016/j.biombioe.2020.105677 |
| [58] | XIANG Y, SHAO ZY, CHAI HX, JI FY, HE Q. Functional microorganisms and enzymes related nitrogen cycle in the biofilm performing simultaneous nitrification and denitrification. Bioresource Technology, 2020, 314: 123697. DOI:10.1016/j.biortech.2020.123697 |
| [59] | LI CE, YANG JS, WANG X, WANG ET, LI BZ, HE RX, YUAN HL. Removal of nitrogen by heterotrophic nitrification-aerobic denitrification of a phosphate accumulating bacterium Pseudomonas stutzeri YG-24. Bioresource Technology, 2015, 182: 18-25. DOI:10.1016/j.biortech.2015.01.100 |
| [60] | PAL RR, KHARDENAVIS AA, PUROHIT HJ. Identification and monitoring of nitrification and denitrification genes in Klebsiella pneumoniae EGD-HP19-C for its ability to perform heterotrophic nitrification and aerobic denitrification. Functional & Integrative Genomics, 2015, 15(1): 63-76. |
| [61] | CASKEY WH, TIEDJE JM. The reduction of nitrate to ammonium by a Clostridium sp. isolated from soil. Journal of General Microbiology, 1980, 119(1): 217-223. |
| [62] | ZHOU ZM, TAKAYA N, NAKAMURA A, YAMAGUCHI M, TAKEO K, SHOUN H. Ammonia fermentation, a novel anoxic metabolism of nitrate by fungi. The Journal of Biological Chemistry, 2002, 277(3): 1892-1896. DOI:10.1074/jbc.M109096200 |
| [63] | DING XH, LAN WS, LI YL, YAN AX, KATAYAMA Y, KOBA K, MAKABE A, FUKUSHIMA K, YANO M, ONISHI Y, GE QY, GU JD. An internal recycling mechanism between ammonia/ammonium and nitrate driven by ammonia-oxidizing Archaea and bacteria (AOA, AOB, and Comammox) and DNRA on Angkor sandstone monuments. International Biodeterioration & Biodegradation, 2021, 165: 105328. |
| [64] | LAM P, KUYPERS MMM. Microbial nitrogen cycling processes in oxygen minimum zones. Annual Review of Marine Science, 2011, 3(1): 317-345. DOI:10.1146/annurev-marine-120709-142814 |
| [65] | HE TX, XIE DT, NI JP, LI Z, LI ZL. Characteristics of nitrogen transformation and intracellular nitrite accumulation by the hypothermia bacterium Arthrobacter arilaitensis. Science of the Total Environment, 2020, 701: 134730. DOI:10.1016/j.scitotenv.2019.134730 |
| [66] | HE TX, CHEN MP, DING CY, WU QF, ZHANG MM. Hypothermia Pseudomonas taiwanensis J488 exhibited strong tolerance capacity to high dosages of divalent metal ions during nitrogen removal process. Bioresource Technology, 2021, 341: 125785. DOI:10.1016/j.biortech.2021.125785 |
| [67] | SONG T, ZHANG XL, LI J, WU XY, FENG HX, DONG WY. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Science of the Total Environment, 2021, 801: 149319. DOI:10.1016/j.scitotenv.2021.149319 |
| [68] | JIN P, CHEN YY, YAO R, ZHENG ZW, DU QZ. New insight into the nitrogen metabolism of simultaneous heterotrophic nitrification-aerobic denitrification bacterium in mRNA expression. Journal of Hazardous Materials, 2019, 371: 295-303. DOI:10.1016/j.jhazmat.2019.03.023 |
| [69] | HANSON TE, CAMPBELL BJ, KALIS KM, CAMPBELL MA, KLOTZ MG. Nitrate ammonification by Nautilia profundicola AmH: experimental evidence consistent with a free hydroxylamine intermediate. Frontiers in Microbiology, 2013, 4: 180. |
| [70] | KOSTERA J, MCGARRY J, PACHECO AA. Enzymatic interconversion of ammonia and nitrite: the right tool for the job. Biochemistry, 2010, 49(39): 8546-8553. DOI:10.1021/bi1006783 |
| [71] | BURLAT B, GWYER JD, POOCK S, CLARKE T, COLE JA, HEMMINGS AM, CHEESMAN MR, BUTT JN, RICHARDSON DJ. Cytochrome c nitrite reductase: from structural to physicochemical analysis. Biochemical Society Transactions, 2005, 33(Pt 1): 137-140. |
| [72] |
NIE M, LI ZL. Bioprocess of nitrite accumulation in water—a review. Chinese Journal of Biotechnology, 2020, 36(8): 1493-1503.
(in Chinese) 聂铭, 李振轮. 水体中亚硝酸盐积累的生物过程及影响因素研究进展. 生物工程学报, 2020, 36(8): 1493-1503. |
| [73] |
WU QF, DING CY, HE TX, CHEN MP, ZHANG MM. Research progress of hydroxylamine oxidoreductase in biological denitrification. Microbiology China, 2022, 49(3): 1120-1134.
(in Chinese) 吴启凤, 丁晨雨, 何腾霞, 陈梦苹, 张漫漫. 羟胺氧化还原酶在生物脱氮中的研究进展. 微生物学通报, 2022, 49(3): 1120-1134. |
| [74] | HAASE D, HERMANN B, EINSLE O, SIMON J. Epsilonproteobacterial hydroxylamine oxidoreductase (εHao): characterization of a 'missing link' in the multihaem cytochrome c family. Molecular Microbiology, 2017, 105(1): 127-138. |
| [75] | KOSTERA J, YOUNGBLUT MD, SLOSARCZYK JM, PACHECO AA. Kinetic and product distribution analysis of NO reductase activity in Nitrosomonas europaea hydroxylamine oxidoreductase. Journal of Biological Inorganic Chemistry, 2008, 13(7): 1073-1083. |
| [76] | ZHAO YY, LI QX, CUI QJ, NI SQ. Nitrogen recovery through fermentative dissimilatory nitrate reduction to ammonium (DNRA): carbon source comparison and metabolic pathway. Chemical Engineering Journal, 2022, 441: 135938. |
| [77] | XI HP, ZHOU XT, ARSLAN M, LUO ZJ, WEI J, WU ZR, GAMAL EL-DIN M. Heterotrophic nitrification and aerobic denitrification process: promising but a long way to go in the wastewater treatment. Science of the Total Environment, 2022, 805: 150212. |
| [78] | SU ZQ, ZHANG Y, JIA X, XIANG XM, ZHOU JT. Research on enhancement of zero-valent iron on dissimilatory nitrate/nitrite reduction to ammonium of Desulfovibrio sp. CMX. The Science of the Total Environment, 2020, 746: 141126. |
| [79] | XIE FX, THIRI M, WANG H. Simultaneous heterotrophic nitrification and aerobic denitrification by a novel isolated Pseudomonas mendocina X49. Bioresource Technology, 2021, 319: 124198. |
| [80] | SUN YH, DE VOS P, WILLEMS A. Influence of nitrate and nitrite concentration on N2O production via dissimilatory nitrate/nitrite reduction to ammonium in Bacillus paralicheniformis LMG 6934. MicrobiologyOpen, 2018, 7(4): e00592. |
| [81] | WU LH, DING XY, LIN Y, LU XS, LV H, ZHAO MP, YU RH. Nitrogen removal by a novel heterotrophic nitrification and aerobic denitrification bacterium Acinetobacter calcoaceticus TY1 under low temperatures. Bioresource Technology, 2022, 353: 127148. |
| [82] | MA S, HUANG SW, TIAN Y, LU XY. Heterotrophic ammonium assimilation: an important driving force for aerobic denitrification of Rhodococcus erythropolis strain Y10. Chemosphere, 2022, 291(Pt 3): 132910. |
| [83] | ZHANG HH, MA B, HUANG TL, SHI YJ. Nitrate reduction by the aerobic denitrifying actinomycete Streptomyces sp. XD-11-6-2:performance, metabolic activity, and micro-polluted water treatment. Bioresource Technology, 2021, 326: 124779. |
| [84] | KRAFT B, TEGETMEYER HE, SHARMA R, KLOTZ MG, FERDELMAN TG, HETTICH RL, GEELHOED JS, STROUS M. Nitrogen cycling. The environmental controls that govern the end product of bacterial nitrate respiration. Science, 2014, 345(6197): 676-679. |
| [85] | HUANG XJ, TIE WZ, XIE DT, LI ZL. Low C/N ratios promote dissimilatory nitrite reduction to ammonium in Pseudomonas putida Y-9 under aerobic conditions. Microorganisms, 2021, 9(7): 1524. |
| [86] | RIBAS D, CALDERER M, MARTÍ V, ROVIRA M. Effect of different seasonal conditions on the potential of wetland soils for groundwater denitrification. Desalination and Water Treatment, 2015, 53(4): 994-1000. |
| [87] | XIANG SY, LIU YH, ZHANG GM, RUAN R, WANG YP, WU XD, ZHENG HL, ZHANG Q, CAO LP. New progress of ammonia recovery during ammonia nitrogen removal from various wastewaters. World Journal of Microbiology & Biotechnology, 2020, 36(10): 144. |
| [88] |
LIU J. Preliminary research on aerobic and dissimilatory nitrate reduction to ammonium by bacteria[D]. Chengdu: Master's Thesis of Sichuan University, 2007 (in Chinese). 刘佳. 微生物好氧硝酸盐还原产铵研究[D]. 成都: 四川大学硕士学位论文, 2007. |
| [89] | ZHANG T, FANG C, LI P, JIANG RF. Nutrient recovery from piggy wastewater by enhancing struvite crystallization process. Applied Mechanics and Materials, 2014, 3013(522/523/524): 579-583. |
| [90] | WANG SB, PENG YL. Natural zeolites as effective adsorbents in water and wastewater treatment. Chemical Engineering Journal, 2010, 156(1): 11-24. |
| [91] | ATA ON, KANCA A, DEMIR Z, YIGIT V. Optimization of ammonia removal from aqueous solution by microwave-assisted air stripping. Water, Air, & Soil Pollution, 2017, 228(11): 1-10. |
| [92] | ZHANG XY, LIU Y. Circular economy-driven ammonium recovery from municipal wastewater: state of the art, challenges and solutions forward. Bioresource Technology, 2021, 334: 125231. |
| [93] | JEONG D, LIM H, KO MS, KIM W. Utilization of three-layered polyvinyl alcohol gel cubes for treating low-strength ammonium wastewater in a single-stage autotrophic nitrogen removal process. Journal of Environmental Chemical Engineering, 2022, 10(1): 106934. |
| [94] | ZHANG L, LEE YW, JAHNG D. Ammonia stripping for enhanced biomethanization of piggery wastewater. Journal of Hazardous Materials, 2012, 199/200: 36-42. |
| [95] | ARREDONDO MR, KUNTKE P, JEREMIASSE AW, SLEUTELS THJA, BUISMAN CJN, TER HEIJNE A. Bioelectrochemical systems for nitrogen removal and recovery from wastewater. Environmental Science: Water Research & Technology, 2015, 1(1): 22-33. |
| [96] | YELLEZUOME D, ZHU XP, WANG ZZ, LIU RH. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: a review. Renewable and Sustainable Energy Reviews, 2022, 157: 112043. |
 2023, Vol. 63
2023, Vol. 63