
中国科学院微生物研究所,中国微生物学会
文章信息
- 段娇, 刘阳, 冯广达, 杨恩, 朱红惠. 2023
- DUAN Jiao, LIU Yang, FENG Guangda, YANG En, ZHU Honghui.
- 柑橘溃疡病及其微生物防治研究进展
- Research progress on citrus canker disease and its microbial control
- 微生物学报, 63(5): 1944-1958
- Acta Microbiologica Sinica, 63(5): 1944-1958
-
文章历史
- 收稿日期:2023-03-06
- 网络出版日期:2023-04-25
2. 广东省科学院微生物研究所 华南应用微生物国家重点实验室 农业农村部农业微生物组学与精准应用重点实验室 农业农村部农业微生物组学重点实验室 广东省菌种保藏与应用重点实验室, 广东 广州 510070
2. Key Laboratory of Agricultural Microbiomics and Precision Application (Ministry of Agriculture and Rural Affairs), Guangdong Provincial Key Laboratory of Microbial Culture Collection and Application, Key Laboratory of Agricultural Microbiome (Ministry of Agriculture and Rural Affairs), State Key Laboratory of Applied Microbiology Southern China, Institute of Microbiology, Guangdong Academy of Sciences, Guangzhou 510070, Guangdong, China
柑橘系统分类是芸香科、柑橘属植物,性喜温暖湿润气候,包括柑类、橘类、橙类、柚类和柠檬类等品种[1]。柑橘是世界第一大类水果,更是我国种植面积最大、产量最高和经济地位最重要的果树[2]。柑橘类水果富含维生素、果胶和有机酸等营养成分,具有抗菌、抗肿瘤、抗炎和抗氧化等作用,深受人们喜爱[3]。目前,影响柑橘产量和品质的侵染性病虫害主要包括溃疡病、黄龙病、炭疽病、灰霉病、黄脉病、青霉病、绿霉病、木虱、潜叶蛾和红蜘蛛等,其中溃疡病是最具破坏性细菌性病害之一[4-6]。柑橘溃疡病严重危害柑橘的生产和贸易,柑橘一旦感染溃疡病将终生带毒,防治难度极大,给柑橘产业造成巨大的经济损失。
目前,柑橘溃疡病防控措施主要包括种植抗病或对溃疡病不敏感的柑橘品种,清除染病苗木,定期检查和修剪果树,建立防风林,定期喷洒铜基杀菌剂、抗生素以及喷施杀虫剂等[4, 7-10]。其中,以铜基杀菌剂为代表的化学杀菌剂当前应用最广泛,防治效果最明显。但是,长期使用化学杀菌剂易导致土壤、水体污染,农药残留,耐抗药性等问题[7, 11-14]。因此,探索绿色、高效的柑橘溃疡病新型防治方法迫在眉睫。近年来,具有广谱、高效、环境相容性好等优点的微生物防治方法引起了国内外广泛关注。
微生物防治是指通过微生物、活性物质等抑制和(或)杀死病原菌的防治方法。目前,微生物防治柑橘溃疡病的研究逐渐增多。本文主要从柑橘溃疡病特征,病原菌及其致病机理、柑橘溃疡病生防微生物多样性及作用机制、柑橘溃疡病目前防治存在的问题和后续研究方向等4个方面进行概述,以期通过对柑橘溃疡病及其微生物防治研究进展的总结为柑橘产业健康发展提供参考。
1 柑橘溃疡病及其病原菌柑橘作为人工培育植物品种之一,原产于中国南部,随后传播到多个国家和地区[1]。在柑橘生产和贸易过程中,柑橘溃疡病危害极大,也是我国柑橘产区最严重病害之一。柑橘溃疡病病原菌主要是柑橘黄单胞菌柑橘亚种,Xanthomonas citri subsp. citri (Xcc)[15-19]。Xcc感染柑橘后,叶片、枝条和果实等器官会出现溃疡症状,而后逐渐形成溃疡病斑。感染初期,受害叶片背面出现淡黄色油渍状小斑点,继而发展成近圆形褐色病斑;随后感染位点隆起突出,病斑中心破裂下陷,呈溃疡状[20-24];枝条和果实的病斑与叶片相似。柑橘溃疡病导致落叶、落果、枝条干枯,严重时甚至引起苗木死亡[20-24]。高温多雨条件易发生柑橘溃疡病,Xcc通过气孔、水孔、皮孔或伤口等感染柑橘[10];Xcc也能够通过苗木、枝条和果实等远距离传播[21]。因此,通过合理施肥用药、严控害虫和根除发病苗木等措施能有效降低柑橘溃疡病的发生。
Xcc隶属于假单胞菌门、γ-变形菌纲、溶杆菌科、黄单胞菌属[21, 23]。在过去很长一段时间,由于认识和技术局限,包含Xcc在内的黄单胞菌类群多个近缘物种的分类关系发生多次变动。最近,系统基因组学研究已重构了黄单胞菌类群的分类体系,确定了Xcc分类地位[25]。Xcc,革兰氏染色阴性,细胞呈杆状,好氧生长,利用单端生鞭毛运动,能够滑动和泳动,在固体表面特别是柑橘溃疡发生部位易形成生物膜[21, 23]。Xcc耐干旱和低温,在宿主器官可存活数月,因此极难被清除。不同柑橘品种对Xcc的敏感程度不同,其发病率通常随着器官成熟度的增加而降低[26]。根据宿主范围和对柑橘敏感程度等差异,柑橘溃疡病病原菌可划分为3个致病型,A、B和C[27-28]。致病型A对柑橘的致病性最高,破坏性最大,包括XccAw和XccA*两种变异体,XccAw主要分布于美洲,XccA*主要分布于亚洲和非洲,两种变异体的效应分子不同[18, 29]。致病型B和C的病原菌是Xanthomonas fuscans subsp. aurantifolii,前者分布于南美洲,后者仅分布于巴西[27]。致病型B和C通常被认为是致病型A的减(弱)毒株,其宿主范围、分布区域、危害程度显著低于致病型A[27]。因此,Xcc致病型A是柑橘溃疡病防治重点,是本文重点阐述的对象。
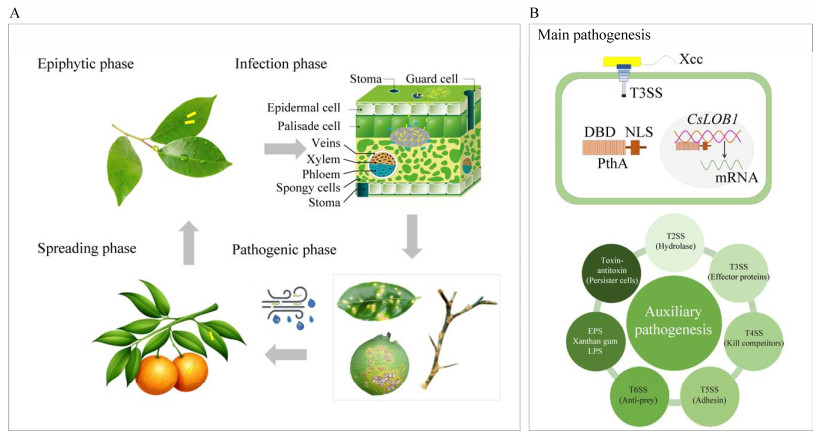
2 病原菌Xcc致病机理柑橘溃疡病危害柑橘叶片、枝条和果实。在柑橘个体和器官水平,柑橘溃疡病发病周期相似。根据病害发生发展过程,柑橘溃疡病发病周期可划分为4个时期:(1) 附生期(epiphytic phase):Xcc附着在柑橘器官表面,开始生长增殖。在此期间,为了抵御环境压力和生物胁迫,Xcc通过分泌黏附素和胞外聚合物等形成具有保护作用的生物膜。(2) 感染期(infection phase):高温、高湿、多风雨条件下,Xcc通过自然孔口或伤口侵入柑橘器官细胞间隙和栅栏组织,进行定殖。(3) 致病期(pathogenic phase):Xcc感染叶片、枝条和果实后会产生溃疡病斑。叶片症状始于背面黄色油渍状斑点,接着病斑穿透叶片,形成两面突出,呈海绵状,组织木栓化,随后病斑中央凹陷,呈火山口状开裂。枝条和果实的病斑与叶片相似,但面积更大,木栓化程度更高。(4) 扩散期(spreading phase):溃疡病病斑开裂后,Xcc在风雨、昆虫、枝叶接触或人为因素等作用下释放到周围环境和其他健康植株,在适合条件下开始新发病周期(图 1A)。
|
| 图 1 柑橘溃疡病发病周期(A)及致病机理(B) Figure 1 The pathogenetic cycle (A) and mechanism (B) of citrus canker disease. |
在细胞和分子水平,柑橘溃疡病形成的原因是Xcc产生的毒力因子与柑橘感病基因相互作用,进而激活感病基因转录,引起柑橘侵染部位细胞的病理性增生膨大,产生溃疡病症状。同时,Xcc能够产生效应蛋白、毒力因子、扩散信号分子,形成附属结构,提高其在柑橘器官的生存能力,进而增强其致病性[30]。
2.1 主要致病机理Xcc基因组序列包含基因pthA,其侵染柑橘后会产生致病因子PthA。Xcc通过Ⅲ型分泌系统(T3SS)将PthA注入宿主细胞。随后,PthA与柑橘感病基因CsLOB1启动子区域的EBS序列识别、结合,激活基因CsLOB1转录,引起柑橘细胞的增生膨大,形成柑橘溃疡病病变[31-33]。基因pthA是avrBs3基因家族成员,其产物PthA是T3SS效应因子(毒力蛋白),介导蛋白质-蛋白质、蛋白质-DNA相互作用,调节宿主转录[34]。在PthA蛋白与宿主互作过程中,PthA通过Xcc的T3SS进入植物细胞,其核定位信号区域与宿主细胞Importin α结合,在Importin β协同作用下,将PthA引向植物细胞核[35]。在细胞核中,PthA中由内部重复形成的DNA结合结构域和基因CsLOB1启动子区域的效应因子结合位点序列识别和结合,激活基因CsLOB1转录[36]。基因CsLOB1属于植物侧翼器官边界转录因子家族成员,该家族主要参与植物侧生器官发育,在植物生长发育中发挥关键作用。基因CsLOB1表达与细胞增殖有关,其转录激活将引起宿主细胞病理性增生、膨大和坏死,这是柑橘溃疡病在细胞水平的典型症状[31]。基因CsLIEXP1为PthA4间接诱导的易感基因,是基因CsLOB1的直接靶标[37]。PthA也能够与柑橘CsCYP、CsMAF1和CsCAF1等因子发生相互作用。PthA与CsMAF1结合能够释放RNA聚合酶Ⅲ,促进核糖体和蛋白质合成[38]。PthA能够抑制CsCAF1活性,稳定基因CsLOB1编码的mRNA[39]。Xcc产生的PthA是诱发柑橘溃疡病的关键激活因子,柑橘感病基因CsLOB1是柑橘溃疡病产生的主要效应因子(图 1B)。因此,柑橘溃疡病主要致病机理(main pathogenesis)由Xcc和柑橘组成的PthA-T3SS-CsLOB1系统介导。
2.2 协助致病机理Xcc也可以利用多种分泌系统,产生胞外聚合物,通过群体感应等途径增强其致病性(图 1B),因此被称为Xcc协助致病机理(auxiliary pathogenesis)。
Xcc通过多种分泌系统的协同作用进而增强其侵染和定殖能力。Ⅱ型分泌系统(T2SS)分泌纤维素酶、几丁质酶等水解酶类(hydrolase),降解宿主细胞壁及细胞内容物,消除宿主的机械屏障,从而促进Xcc侵染柑橘;降解产物也能够为Xcc生长增殖提供营养物质[40]。除了PthA,T3SS也能够产生其他类型效应蛋白(effector proteins),干扰宿主细胞膜的识别能力,对抗宿主自身免疫系统。与Ⅳ型分泌系统(T4SS)密切相关的菌毛则有助于Xcc的附着、移动和生物膜形成[41];Xcc也能够通过T4SS以接触依赖方式杀死紧邻的革兰氏阴性菌(kill competitors),从而获得竞争优势[42]。V型分泌系统(T5SS)分泌的黏附蛋白(adhesin) FhaB介导宿主的黏附,在Xcc感染早期阶段发挥重要作用[43-45]。Xcc能够利用Ⅵ型分泌系统(T6SS)避免被黏菌捕食(Anti-prey)[46]。同时,Xcc通过产生胞外聚合物,提高其与宿主的结合能力,增强其环境适应性,促进其侵染、生存生活、增殖和致病等。胞外多糖(exopolysaccharides, EPS)和脂多糖(lipopolysaccharides, LPS)能够降低细胞膜通透性,保护Xcc免受植物组织内相关物质影响,从而提高其生存能力[30, 47]。Xcc产生的黄原胶(xanthan gum)能够促进其在宿主中存活,也能够阻挡宿主导管对水的运输,导致宿主枯萎死亡[30, 48-49]。另外,Xcc通过群体感应,形成持留细胞,产生菌黄素等途径增强其致病性。扩散性信号分子介导的群体感应(QS)能够特异性调控Xcc运动、毒力因子等相关基因表达,促进其感染宿主[30, 50]。Xcc可以通过毒素-抗毒素系统(toxin-antitoxin)形成持留细胞(persister cells),长期存活[51];产生菌黄素,抵抗光氧化伤害,促进其在植物器官表面的附着、生长[52];感知柑橘叶片质外体渗出物,促进其对宿主的侵染[53];其鞭毛蛋白XaFliC可作为有效防御激发子,诱发柑橘免疫系统抗性[54]。
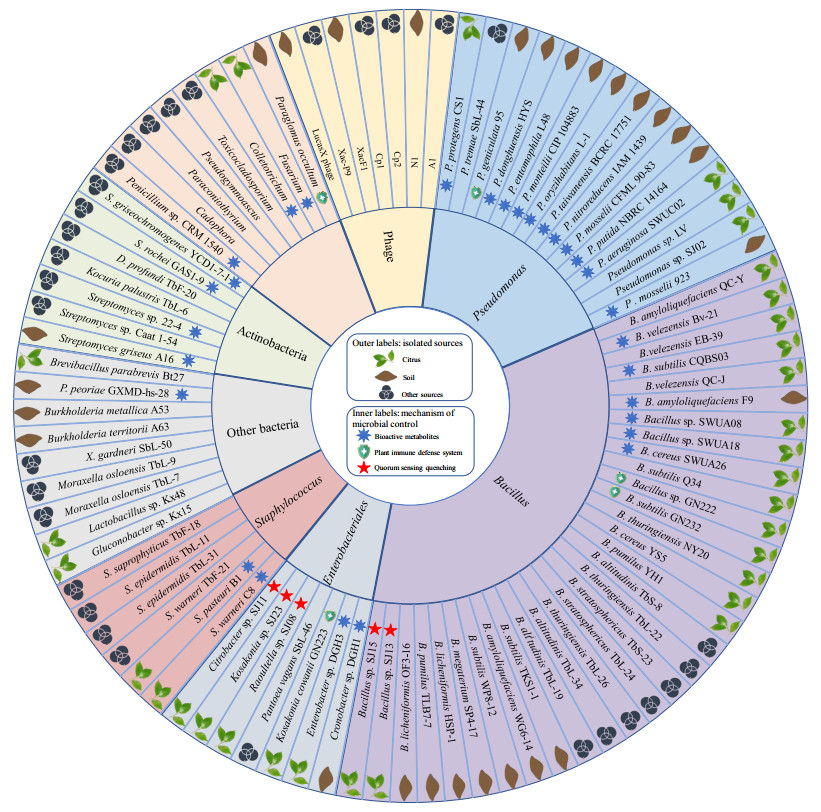
3 柑橘溃疡病生防微生物多样性与化学防治易污染环境、破坏生态平衡相比,利用微生物进行柑橘溃疡病的防治具有高效、绿色、不产生抗性等优点。按照种类差异,柑橘溃疡病生防微生物可划分为生防细菌、生防真菌和生防噬菌体(图 2,附表1已提交至国家微生物科学数据中心,编号为NMDCX0000195)。
|
| 图 2 柑橘溃疡病生防微生物的分类、来源及作用机制 Figure 2 Taxonomy, isolated sources and biocontrol mechanisms of microorganisms against citrus canker disease. |
3.1 生防细菌
柑橘溃疡病生防细菌是当前研究最多、分类最细和应用最广的类群,主要包括芽胞杆菌、假单胞菌和放线菌等,其中前两者报道最多。芽胞杆菌生态分布广泛,抗逆性能强,能够产生多种抗菌活性物质,被认为是最具应用潜力的生防细菌。目前报道的对柑橘溃疡病具有拮抗作用的芽胞杆菌主要包括枯草芽胞杆菌、解淀粉芽胞杆菌和贝莱斯芽胞杆菌等。Qian等研究发现脐橙叶片来源的菌株Bacillus amyloliquefaciens QC-Y能够显著降低柑橘溃疡病发病率及叶片病斑面积[19]。Rabbee等研究发现21株芽胞杆菌乙酸乙酯提取物均具有拮抗Xcc的活性,其中Bacillus velezensi BV-21抑菌率最高[55]。假单胞菌对柑橘溃疡病的生防作用也是研究热点。Villamizar等首次报道Pseudomonas entomophila JS2对柑橘溃疡病具有较好防治效果[56]。Michavila等研究发现分离于柠檬叶片的菌株Pseudomonas protegens CS1体外、叶片测试均能抑制Xcc生长[57]。值得注意的是,许多假单胞菌是条件致病菌,后续应用时应特别关注其安全性。相对于芽胞杆菌和假单胞菌,放线菌类群的生防细菌则报道较少,且多为链霉菌。董玉兰等研究发现土壤来源的链霉菌CLT3在温室内对Xcc防治效果达到83.85%[58]。另外,少数肠杆菌、乳酸菌和葡萄球菌等也具有防治柑橘溃疡病的效果。基于分离来源,目前报道的生防细菌主要分离于柑橘器官(叶片、果实等)及其生长环境(根际土壤)。
3.2 生防真菌真菌在柑橘叶际、根际和周围土壤广泛存在,且多样性丰富。许多真菌能够增强柑橘对溃疡病的抗性,因此常用于柑橘溃疡病的防治。与普通环境相比,极端环境来源的真菌具有特殊的遗传背景、代谢途径,能够产生更多新颖活性物质,作用于Xcc。Vieira等研究发现南极土壤和海洋沉积物来源的具有抑制Xcc活性的真菌属于Pseudogymnoascus、Penicillium、Cadophora、Paraconiothyrium和Toxicocladosporium,其次级代谢产物对Xcc的平均抑制率分别为94和97%[59]。该团队随后发现菌株Penicillium sp. CRM 1540产生的Penicillic acid在25 μg/mL浓度下对柑橘溃疡病的体外抑制率高达90%[60]。Xie等研究表明接种丛枝菌根真菌能够显著提高柑橘对Xcc的抗性[61]。颜桢灵等从健康柑橘分离鉴定了72株内生真菌,其中29株真菌的乙酸乙酯提取物对Xcc具有抑制活性[62]。总体而言,与细菌拮抗Xcc研究比较,目前生防真菌的研究较少,后续开发利用的潜力巨大。
3.3 生防噬菌体噬菌体宿主特异性强,可识别特定病原菌,对环境影响小;能够在宿主细胞内大量增殖,高效裂解病原菌;与宿主协同进化,可实现对病原菌的动态控制。因此,利用噬菌体防治Xcc是一种绿色生态技术。Ali Ahmad等研究发现土壤来源的丝状噬菌体XacF1能够整合至Xcc基因组,引起Xcc胞外多糖产量降低,运动能力减弱,生长速度变慢,进而降低Xcc毒力[63]。随后其在鉴定具有裂解Xcc噬菌体的过程中发现,噬菌体Cp1和Cp2能够利用Xcc细胞表面不同
分子作为受体,附着在宿主细胞[64]。2018年,Yoshikawa等从日本柑橘园土壤分离获得了1株能够感染Xcc且宿主更广泛的巨型噬菌体N1[65]。Balogh等研究发现噬菌体CP2和ΦXac2005-1等能够降低柑橘溃疡病发病率,而噬菌体ΦXacF1能够显著减弱葡萄柚溃疡病的发生[66]。Ibrahim等利用噬菌体和阿拉酸式-S-甲基混合物处理被Xcc侵染的墨西哥青柠,在温室中发病率降低为18.3%,大田灌根发病率降低至15.8%[67]。肖逍等从污水分离获得了1株针对Xcc的烈性噬菌体Xac-P9,其对17株不同来源Xac裂解率为100%,30 min可杀灭90%宿主,150 min可以杀死100%宿主,且其对温度和pH具有耐受性,对紫外线辐射不敏感,具有较好应用潜力[68]。噬菌体能够特异裂解Xcc,对环境友好,是一种替代铜基杀菌剂和抗生素的绿色微生物防治方法。但是,目前报道能够裂解Xcc的噬菌体数量和种类有限。另外,噬菌体对温、热、光等环境因素敏感,长期使用可能会产生抗性问题;一些噬菌体也可能介导耐药基因和抗性基因的水平转移。因此,后续通过噬菌体修饰、载体负载、噬菌体鸡尾酒疗法和噬菌体——抗生素联合使用等方法能够提高噬菌体生防效果。
4 柑橘溃疡病生防微生物作用机制柑橘溃疡病生防微生物作用机制主要包括3个方面:(1) 产生的活性物质通过破坏Xcc生理结构、代谢功能而抑制Xcc细胞生长和生物膜形成,限制其侵染能力,降低其致病能力,甚至直接杀死Xcc;(2) 通过诱导激活柑橘自身免疫系统,使其产生对Xcc的系统抗性;(3) 通过抑制Xcc分泌系统、与Xcc竞争营养和生态位、群体感应淬灭、噬菌体裂解和促生等作用机制防治Xcc。
4.1 产生活性物质许多柑橘溃疡病生防微生物通过产生活性物质抑制Xcc在柑橘表面附着定殖,阻止胞外多聚物产生和生物膜形成,破坏细胞壁和细胞膜,甚至杀死Xcc。目前已报道的活性物质主要包括肽类、蛋白酶和铁载体等。Wattana-Amorn等发现菌株Streptomyces sp. 22-4产生的环二肽cyclo(L-Pro-L-Tyr)和cyclo(D-Pro-L-Tyr)对Xcc具有抑制作用,其最小抑菌浓度是31.25 μg/mL[69]。Habibollahi等研究表明纯化的重组多肽CAP18在4.5 μmol/L浓度下对Xcc的抑制率为90%,其抑菌作用是重组多肽渗透至Xcc细胞膜的磷脂双分子层,进而引起细胞膜变薄和形变[70]。Wang等研究确定菌株Bacillus amyloliquefaciens F9分泌的3种脂肽类化合物包括Surfactin、Fengycin和Iturin能够抑制Xcc产生胞外酶,诱导Xcc细胞壁裂解[71]。陈力等研究发现菌株Bacillus subtilis CQBS03抑菌物质的主要成分是外泌型蛋白,其对Xcc抑菌活性高,稳定性好[72]。Michavila等对菌株Pseudomonas protegens CS1发酵产物进行鉴定,结果表明其能够分泌产生铁载体Enantio-pyochelin,其通过诱导活性氧自由基引起的氧化胁迫抑制Xcc生长[57]。陈功友团队发现菌株Pseudomonas mosselii 923产生的次级代谢产物Pseudoiodinine对包括Xcc在内的多株Xanthomonas有明显拮抗作用,并解析了该物质的合成途径与调控机制[73]。Vieira等研究确定菌株Penicillium sp. CRM 1540拮抗Xcc的主要活性成分是Penicillic acid[60]。
4.2 诱导激活植物防御系统生防微生物通过产生多种物质,诱导激活柑橘免疫防御系统,进而增强其免疫力和抗病性,减轻甚至消除Xcc危害。Ramos等通过在柑橘叶片喷洒Bacillus subtilis、生物活性铜、氢氧化铜和Acibenzolar-S-methyl,进行柑橘系统获得性抗性激活研究,发现Bacillus subtilis能降低柑橘溃疡病的发病率[74]。赖家豪等研究发现3株内生细菌Bacillus sp. GN222、Kosakonia cowanii GN223和Bacillus subtilis GN232均可诱导并提高脐橙体内防御酶活性,进而增强脐橙抵抗Xcc侵染[75]。Xie等研究发现与未接种丛植菌根真菌的柑橘相比,Xcc侵染后接种丛植菌根真菌,柑橘根部的过氧化氢、一氧化氮、钙调蛋白和水杨酸等信号物质明显增加,防御基因PtEPS1和PtPR4表达水平显著上调,表明接种菌根真菌的柑橘能够通过增强信号底物积累和提高病原菌防御基因表达等方式增强对Xcc抗性[61]。Riera等研究确定3株根际细菌Burkholderia territorii A63、Burkholderia metallica A53和Pseudomonas geniculata 95能够诱导激活柑橘防御系统,减轻叶片溃疡病症状[76],并发现根施P. geniculata能够诱导水杨酸信号通路标记基因和水杨酸羧甲基转移酶表达,增加水杨酸羧甲基转移酶和苯丙氨酸脱氨酶编码基因的转录水平,进而通过提高柑橘免疫活性增强对Xcc抗性[76]。
4.3 其他作用机制生防微生物通过竞争作用、抑制分泌系统、群体感应淬灭和噬菌体裂解等途径防治Xcc。生防微生物与Xcc竞争营养、水分、微量元素、氧气和空间位点等,抑制或杀死Xcc,从而降低Xcc危害,比如芽胞杆菌通过分泌铁载体限制病原体对铁的吸收从而抑制病原体[77-80]。生防微生物通过抑制Xcc与产生毒力因子的相关分泌系统,比如抑制T3SS阻止PthA侵入宿主细胞,通过对T4SS的抑制而影响其菌毛,从而对Xcc吸附定殖造成影响,降低柑橘溃疡病的发生[81]。群体感应是细菌感应群体密度,调控基因表达的一种保守调控机制,与病原菌生物膜形成、细胞运动、毒力因子产生、毒素分泌和耐药性等密切相关[82]。因此,群体感应可以作为细菌病原菌防治靶点,通过影响信号分子合成、降解信号分子、阻止信号分子与受体蛋白结合等途径阻断群体感应,即群体感应淬灭[83-85]。Caicedo等研究发现来源于柑橘叶片的3株细菌Bacillus sp. SJ13、Pseudomonas sp. SJ02和Pseudomonas sp. SJ01通过降解扩散性信号分子扰乱Xcc群体感应,阻止其生物膜形成,进而减弱柑橘溃疡病症状[50]。相较于群体感应淬灭,噬菌体裂解则是一种直接、简便的Xcc防治方法。噬菌体吸附器官与宿主表面受体结合,通过尾轴将其DNA注入宿主体内,利用宿主进行DNA复制和蛋白质合成以及噬菌体组装,最终导致宿主细胞裂解死亡[68]。噬菌体特异性强,生物安全性高,呈指数传播,作为生防菌体,极具开发潜力。
5 结语与展望目前,许多优异的柑橘溃疡病生防微生物被报道,但其种类和数量仍然有限,后续应创新分离方法,获取更多高效Xcc生防微生物。利用微生物群落共现性网络分析和机器学习等方法,确定与Xcc显著负相关菌株,通过反向基因组、单细胞筛选、荧光标记等方法开展目标菌株的定向分离。本团队利用高通量分离、快速精准鉴定等方法从柑橘叶际、根际获得一千余株细菌,包括多株高效Xcc拮抗菌株和新物种,初步建立了Xcc拮抗菌株资源库[86-88]。在挖掘Xcc生防微生物资源过程中,选择柑橘叶片作为筛选样品将有助于解决生防微生物叶片定殖和适应性等问题,特别是叶片内生菌作为生防微生物的潜力更大。同时,当前研究主要聚焦于生防微生物的分离鉴定和实验室条件下对Xcc拮抗测试,对其拮抗作用机制的研究有待加强。一些生防微生物的活性物质被鉴定,但更多对Xcc具有抑制活性的粗取物中有效成分及化合物则有待解析。另外,需要更加全面深入探究Xcc诱发柑橘溃疡病的致病机理及生防微生物作用机制,为微生物防治Xcc提供更多目标靶点。
柑橘溃疡病的微生物防治是一个复杂的过程,生防微生物、柑橘、Xcc、叶际土著微生物等生物因素和水、光、热等气候条件均影响其防治效果。目前研究主要是基于单一微生物防治柑橘溃疡病,存在田间稳定性差、作用周期短等问题。因此,需要深入研究生防微生物的田间生理状态、与生物因素的相互作用、对环境变化的响应等。鉴于柑橘溃疡病防治的复杂性,基于微生物群落生态学原理,通过构建合成功能菌群防治Xcc值得关注。总之,微生物防治柑橘溃疡病的研究目前仍处于初级阶段,深入研究Xcc致病机理,挖掘更多生防微生物资源,解析其生防作用机制仍是推动柑橘溃疡病微生物防治实践的重要基础。深入开展活性物质分离鉴定,加强田间试验及安全性评估,优化生防微生物的适配等也是柑橘溃疡病微生物防治领域的重要方向。相信随着柑橘溃疡病及其生防微生物研究的不断深入,将为柑橘产业的可持续发展提供重要保障。
| [1] |
LI XM. Molecular phylogeny of the true citrus fruit trees group (Aurantioideae, Rutaceae) and the origin of cultivated citrus [D]. Chongqing: Doctoral Dissertation of Southwest University, 2010 (in Chinese). 李小孟. 柑橘及其近缘属植物的分子进化与栽培柑橘的起源研究[D]. 重庆: 西南大学博士学位论文, 2010. |
| [2] |
QI CJ, GU YM, ZENG Y. Progress of citrus industry economy in China. Journal of Huazhong Agricultural University, 2021, 40(1): 58-69.
(in Chinese) 祁春节, 顾雨檬, 曾彦. 我国柑橘产业经济研究进展. 华中农业大学学报, 2021, 40(1): 58-69. DOI:10.13300/j.cnki.hnlkxb.2021.01.007 |
| [3] | PEI Y, HE CX, LIU HL, SHEN GP, FENG JH. Compositional analysis of four kinds of Citrus fruits with an NMR-based method for understanding nutritional value and rational utilization: from pericarp to juice. Molecules (Basel, Switzerland), 2022, 27(8): 2579. DOI:10.3390/molecules27082579 |
| [4] | ANGELOTTI-MENDONÇA J, de OLIVEIRA PN, ANSANTE NF, STIPP LCL, FREITAS-ASTÚA J, HURTADO FMM, BELASQUE J, de ASSIS ALVES MOURÃO FILHO F. Expression of the Citrus sinensis EDS5 gene, MATE family, in Solanum lycopersicum L. cv. Micro-Tom enhances resistance to tomato spot disease. Tropical Plant Pathology, 2022, 47(2): 287-297. DOI:10.1007/s40858-021-00480-y |
| [5] | ADHI NY, SUHARJONO S, SRI W. Biological control of Citrus canker pathogen Xanthomonas citri subsp. citri using Rangpur lime endophytic bacteria. Egyptian Journal of Biological Pest Control, 2022, 32(1): 63. DOI:10.1186/s41938-022-00561-3 |
| [6] | GOTTWALD TR, GRAHAM JH, SCHUBERT TS. Citrus canker: the pathogen and its impact[J]. Plant Health Progress, 2002, 3(1). https://doi.org/10.1094/PHP-2002-0812-01-RV. |
| [7] | LONG YF, LUO RF, XU Z, CHENG SY, LI L, MA HJ, BAO ML, LI M, OUYANG ZG, WANG N, DUAN S. A fluorescent reporter-based evaluation assay for antibacterial components against Xanthomonas citri subsp. citri. Frontiers in Microbiology, 2022, 13: 864963. DOI:10.3389/fmicb.2022.864963 |
| [8] | de CARVALHO DU, NEVES CSVJ, da CRUZ MA, LONGHI TV, BEHLAU F, de CARVALHO SA, LEITE RP Jr. Late-season sweet orange selections under huanglongbing and Citrus canker endemic conditions in the Brazilian humid subtropical region. Frontiers in Plant Science, 2022, 13: 915889. DOI:10.3389/fpls.2022.915889 |
| [9] | BEHLAU F, BELASQUE J Jr, LEITE RP Jr, FILHO AB, GOTTWALD TR, GRAHAM JH, SCANDELAI LHM, PRIMIANO IV, BASSANEZI RB, AYRES AJ. Relative contribution of windbreak, copper sprays, and leafminer control for Citrus canker management and prevention of crop loss in sweet orange trees. Plant Disease, 2021, 105(8): 2097-2105. DOI:10.1094/PDIS-10-20-2153-RE |
| [10] | MACHADO FJ, da SILVA MARIN TG, CANÔAS F, da SILVA GJ Jr, BEHLAU F. Timing of copper sprays to protect mechanical wounds against infection by Xanthomonas citri subsp. citri, causal agent of Citrus canker. European Journal of Plant Pathology, 2021, 160(3): 683-692. DOI:10.1007/s10658-021-02276-x |
| [11] | FERREIRA DH, MOREIRA RR, JUNIOR GJS, BEHLAU F. Copper rate and spray interval for joint management of Citrus canker and citrus black spot in orange orchards. European Journal of Plant Pathology, 2022, 163(4): 891-906. DOI:10.1007/s10658-022-02527-5 |
| [12] | CARVALHO CR, DIAS AC, HOMMA SK, CARDOSO EJ. Phyllosphere bacterial assembly in citrus crop under conventional and ecological management. PeerJ, 2020, 8: e9152. DOI:10.7717/peerj.9152 |
| [13] | RICHARD D, BOYER C, LEFEUVRE P, PRUVOST O. Complete genome sequence of a copper-resistant bacterium from the Citrus phyllosphere, Stenotrophomonas sp. strain LM091, obtained using long-read technology. Genome Announcements, 2016, 4(6): e01327-16. |
| [14] | PRUVOST O, RICHARD D, BOYER K, JAVEGNY S, BOYER C, CHIROLEU F, GRYGIEL P, PARVEDY E, ROBÈNE I, MAILLOT-LEBON V, HAMZA A, LOBIN KK, NAIKEN M, VERNIÈRE C. Diversity and geographical structure of Xanthomonas citri pv. citri on Citrus in the south west Indian Ocean region. Microorganisms, 2021, 9(5): 945. DOI:10.3390/microorganisms9050945 |
| [15] | XIAO C, ZHANG H, XIE F, PAN ZY, QIU WM, TONG Z, WANG ZQ, HE XJ, XU YH, SUN ZH. Evolution, gene expression, and protein-protein interaction analyses identify candidate CBL-CIPK signalling networks implicated in stress responses to cold and bacterial infection in citrus. BMC Plant Biology, 2022, 22(1): 420. DOI:10.1186/s12870-022-03809-0 |
| [16] | AMANCIO L, BAIA ADB, SOUZA EB, SALES JÚNIOR R, NEGREIROS AMP, BALBINO VQ, GAMA MAS. First Report of Xanthomonas citri subsp. citri causing Citrus Canker on lime in Rio Grande do Norte, Brazil. Plant Disease, 2021, 105(12): 4148. |
| [17] | REHMAN MSNU. First report of Xanthomonas citri subsp. citri causing Citrus canker on grape fruit (Citrus paradisi), Washington naval (Citrus sinensis), kaghzi Limon (Citrus aurantifolia Swingle), lemon (Citrus lim). Pakistan Journal of Agricultural Sciences, 2021, 58(04): 1373-1377. DOI:10.21162/PAKJAS/21.9701 |
| [18] | LICCIARDELLO G, CARUSO P, BELLA P, BOYER C, SMITH MW, PRUVOST O, ROBENE I, CUBERO J, CATARA V. Pathotyping Citrus ornamental relatives with Xanthomonas citri pv. citri and X. citri pv. aurantifolii refines our understanding of their susceptibility to these pathogens. Microorganisms, 2022, 10(5): 986. DOI:10.3390/microorganisms10050986 |
| [19] | QIAN JL, ZHANG T, TANG S, ZHOU LL, LI KT, FU XQ, YU SJ. Biocontrol of Citrus canker with endophyte Bacillus amyloliquefaciens QC-Y. Plant Protection Science, 2021, 57(1): 1-13. DOI:10.17221/62/2020-PPS |
| [20] | PUCCI N, SCALA V, TATULLI G, L'AURORA A, LUCCHESI S, SALUSTRI M, LORETI S. Intra-laboratory evaluation of DNA extraction methods and assessment of a droplet digital PCR for the detection of Xanthomonas citri pv. citri on different Citrus species. International Journal of Molecular Sciences, 2022, 23(9): 4975. DOI:10.3390/ijms23094975 |
| [21] | GRAHAM JH, GOTTWALD TR, CUBERO J, ACHOR DS. Xanthomonas axonopodis pv. citri: factors affecting successful eradication of Citrus canker. Molecular Plant Pathology, 2004, 5(1): 1-15. DOI:10.1046/j.1364-3703.2004.00197.x |
| [22] | GIRALDO-GONZÁLEZ JJ, de SOUZA CARVALHO FM, FERRO JA, HERAI RH, CHAVES BEDOYA G, RODAS MENDOZA EF. Transcriptional changes involved in kumquat (Fortunella spp) defense response to Xanthomonas citri subsp. citri in early stages of infection. Physiological and Molecular Plant Pathology, 2021, 116: 101729. DOI:10.1016/j.pmpp.2021.101729 |
| [23] | BRUNINGS AM, GABRIEL DW. Xanthomonas citri: breaking the surface. Molecular Plant Pathology, 2003, 4(3): 141-157. DOI:10.1046/j.1364-3703.2003.00163.x |
| [24] | WANG Y, FU XZ, LIU JH, HONG N. Differential structure and physiological response to canker challenge between 'Meiwa' kumquat and 'Newhall' navel orange with contrasting resistance. Scientia Horticulturae, 2011, 128(2): 115-123. DOI:10.1016/j.scienta.2011.01.010 |
| [25] | BANSAL K, KUMAR S, PATIL PB. Phylo-taxonogenomics supports revision of taxonomic status of 20 Xanthomonas pathovars to Xanthomonas citri. Phytopathology®, 2022, 112(6): 1201-1207. DOI:10.1094/PHYTO-08-21-0342-SC |
| [26] | SHARMA A, FERENCE CM, SHANTHARAJ D, BALDWIN EA, MANTHEY JA, JONES JB. Transcriptomic analysis of changes in Citrus × microcarpa gene expression post Xanthomonas citri subsp. citri infection. European Journal of Plant Pathology, 2022, 162(1): 163-181. DOI:10.1007/s10658-021-02394-6 |
| [27] | PATANÉ JSL, MARTINS J Jr, RANGEL LT, BELASQUE J, DIGIAMPIETRI LA, FACINCANI AP, FERREIRA RM, JACIANI FJ, ZHANG YZ, VARANI AM, ALMEIDA NF, WANG N, FERRO JA, MOREIRA LM, SETUBAL JC. Origin and diversification of Xanthomonas citri subsp. citri pathotypes revealed by inclusive phylogenomic, dating, and biogeographic analyses. BMC Genomics, 2019, 20(1): 700. DOI:10.1186/s12864-019-6007-4 |
| [28] | FONSECA NP, FELESTRINO ÉB, CANESCHI WL, SANCHEZ AB, CORDEIRO IF, LEMES CGC, ASSIS RAB, CARVALHO FMS, FERRO JA, VARANI AM, BELASQUE J, SETUBAL JC, TELLES GP, AGUENA DS, ALMEIDA NF, MOREIRA LM. Detection and identification of Xanthomonas pathotypes associated with citrus diseases using comparative genomics and multiplex PCR. PeerJ, 2019, 7: e7676. DOI:10.7717/peerj.7676 |
| [29] | HYUN JW, KIM HJ, YI PH, HWANG RY, PARK EW. Mode of action of streptomycin resistance in the Citrus canker pathogen (Xanthomonas smithii subsp. citri) in jeju island. The Plant Pathology Journal, 2012, 28(2): 207-211. DOI:10.5423/PPJ.2012.28.2.207 |
| [30] | NAQVI SAH, WANG J, MALIK MT, UMAR UUD, ATEEQ-UR-REHMAN, HASNAIN A, SOHAIL MA, SHAKEEL MT, NAUMAN M, HAFEEZ-UR-REHMAN, HASSAN MZ, FATIMA M, DATTA R. Citrus canker—distribution, taxonomy, epidemiology, disease cycle, pathogen biology, detection, and management: a critical review and future research agenda. Agronomy, 2022, 12(5): 1075. DOI:10.3390/agronomy12051075 |
| [31] | ZOU XP, DU MX, LIU YN, WU L, XU LZ, LONG Q, PENG AH, HE YR, ANDRADE M, CHEN SC. CsLOB1 regulates susceptibility to Citrus canker through promoting cell proliferation in citrus. The Plant Journal: for Cell and Molecular Biology, 2021, 106(4): 1039-1057. DOI:10.1111/tpj.15217 |
| [32] | HU Y, ZHANG JL, JIA HG, SOSSO D, LI T, FROMMER WB, YANG B, WHITE FF, WANG N, JONES JB. Lateral organ boundaries 1 is a disease susceptibility gene for citrus bacterial canker disease. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(4): E521-E529. |
| [33] | JIA HG, ORBOVIC V, JONES JB, WANG N. Modification of the PthA4 effector binding elements in Type I CsLOB1 promoter using Cas9/sgRNA to produce transgenic Duncan grapefruit alleviating XccΔpthA4: dCsLOB1.3 infection. Plant Biotechnology Journal, 2016, 14(5): 1291-1301. DOI:10.1111/pbi.12495 |
| [34] | RAEISI H, SAFARNEJAD MR, ALAVI SM, FARROKHI N, ALI ELAHINIA S. Transient expression of an scFvG8 antibody in plants and characterization of its effects on the virulence factor pthA of Xanthomonas citri subsp. citri. Transgenic Research, 2022, 31(2): 269-283. DOI:10.1007/s11248-022-00301-1 |
| [35] | LI N, HUANG L, LIU LP, LI DZ, DAI SM, DENG ZN. The relationship between PthA expression and the pathogenicity of Xanthomonas axonopodis pv. citri. Molecular Biology Reports, 2014, 41(2): 967-975. DOI:10.1007/s11033-013-2941-4 |
| [36] | ABE VY, BENEDETTI CE. Additive roles of PthAs in bacterial growth and pathogenicity associated with nucleotide polymorphisms in effector-binding elements of Citrus canker susceptibility genes. Molecular Plant Pathology, 2016, 17(8): 1223-1236. DOI:10.1111/mpp.12359 |
| [37] | de SOUZA-NETO RR, N C VASCONCELOS F, TEPER D, CARVALHO IGB, TAKITA MA, BENEDETTI CE, WANG N, de SOUZA AA. The expansin gene CsLIEXP1 is a direct target of CsLOB1 in citrus. Phytopathology, 2023. DOI:10.1094/PHYTO-11-22-0424-R |
| [38] | SANTOS SOPRANO A, ABE VY, SMETANA JHC, BENEDETTI CE. Citrus MAF1, a repressor of RNA polymerase Ⅲ, binds the Xanthomonas citri canker elicitor PthA4 and suppresses Citrus canker development. Plant Physiology, 2013, 163(1): 232-242. DOI:10.1104/pp.113.224642 |
| [39] | SHIMO HM, TERASSI C, LIMA SILVA CC, ZANELLA JL, MERCALDI GF, ROCCO SA, BENEDETTI CE. Role of the Citrus sinensis RNA deadenylase CsCAF1 in Citrus canker resistance. Molecular Plant Pathology, 2019, 20(8): 1105-1118. DOI:10.1111/mpp.12815 |
| [40] | SHI Y, YANG XB, YE XX, FENG JY, CHENG TF, ZHOU XF, LIU DX, XU LH, WANG JX. The methyltransferase HemK regulates the virulence and nutrient utilization of the phytopathogenic bacterium Xanthomonas citri subsp. citri. International Journal of Molecular Sciences, 2022, 23(7): 3931. DOI:10.3390/ijms23073931 |
| [41] | DUNGER G, GUZZO CR, ANDRADE MO, JONES JB, FARAH CS. Xanthomonas citri subsp. citri type Ⅳ Pilus is required for twitching motility, biofilm development, and adherence. Molecular Plant-Microbe Interactions®, 2014, 27(10): 1132-1147. DOI:10.1094/MPMI-06-14-0184-R |
| [42] | SOUZA DP, OKA GU, ALVAREZ-MARTINEZ CE, BISSON-FILHO AW, DUNGER G, HOBEIKA L, CAVALCANTE NS, ALEGRIA MC, BARBOSA LRS, SALINAS RK, GUZZO CR, FARAH CS. Bacterial killing via a type Ⅳ secretion system. Nature Communications, 2015, 6: 6453. DOI:10.1038/ncomms7453 |
| [43] | CHOI HW, KIM DS, KIM NH, JUNG HW, HAM JH, HWANG BK. Xanthomonas filamentous hemagglutinin-like protein Fha1 interacts with pepper hypersensitive-induced reaction protein CaHIR1 and functions as a virulence factor in host plants. Molecular Plant-Microbe Interactions: MPMI, 2013, 26(12): 1441-1454. DOI:10.1094/MPMI-07-13-0204-R |
| [44] | GOTTIG N, GARAVAGLIA BS, GAROFALO CG, ORELLANO EG, OTTADO J. A filamentous hemagglutinin-like protein of Xanthomonas axonopodis pv. citri, the phytopathogen responsible for Citrus canker, is involved in bacterial virulence. PLoS One, 2009, 4(2): e4358. DOI:10.1371/journal.pone.0004358 |
| [45] | GARAVAGLIA BS, ZIMARO T, ABRIATA LA, OTTADO J, GOTTIG N. XacFhaB adhesin, an important Xanthomonas citri ssp. citri virulence factor, is recognized as a pathogen-associated molecular pattern. Molecular Plant Pathology, 2016, 17(9): 1344-1353. DOI:10.1111/mpp.12364 |
| [46] | BAYER-SANTOS E, LIMA LDP, CESETI LM, RATAGAMI CY, de SANTANA ES, Da SILVA AM, FARAH CS, ALVAREZ-MARTINEZ CE. Xanthomonas citri T6SS mediates resistance to Dictyostelium predation and is regulated by an ECF σ factor and cognate Ser/Thr kinase. Environmental Microbiology, 2018, 20(4): 1562-1575. DOI:10.1111/1462-2920.14085 |
| [47] | GUO YP, SAGARAM US, KIM JS, WANG N. Requirement of the galU gene for polysaccharide production by and pathogenicity and growth in Planta of Xanthomonas citri subsp. citri. Applied and Environmental Microbiology, 2010, 76(7): 2234-2242. DOI:10.1128/AEM.02897-09 |
| [48] | SENA-VÉLEZ M, GRAHAM JH, GIRÓN JA, REDONDO C, CUBERO J. Characterization of the extracellular matrix of biofilms formed by Xanthomonas citri subsp. citri strains with different host ranges. Tropical Plant Pathology, 2020, 45(3): 306-319. DOI:10.1007/s40858-020-00339-8 |
| [49] | GOLMOHAMMADI M, LLOP P, SCUDERI G, GELL I, GRAHAM JH, CUBERO J. mRNA from selected genes is useful for specific detection and quantification of viable Xanthomonas citri subsp. citri. Plant Pathology, 2012, 61(3): 479-488. DOI:10.1111/j.1365-3059.2011.02526.x |
| [50] | CAICEDO JC, VILLAMIZAR S, FERRO MIT, KUPPER KC, FERRO JA. Bacteria from the citrus phylloplane can disrupt cell-cell signalling inXanthomonas citriand reduce Citrus canker disease severity. Plant Pathology, 2016, 65(5): 782-791. DOI:10.1111/ppa.12466 |
| [51] | MARTINS PMM, WOOD TK, de SOUZA AA. Persister cells form in the plant pathogen Xanthomonas citri subsp. citri under different stress conditions. Microorganisms, 2021, 9(2): 384. DOI:10.3390/microorganisms9020384 |
| [52] | RAJAGOPAL L, SUNDARI CS, BALASUBRAMANIAN D, SONTI RV. The bacterial pigment xanthomonadin offers protection against photodamage. FEBS Letters, 1997, 415(2): 125-128. DOI:10.1016/S0014-5793(97)01109-5 |
| [53] | SENA-VÉLEZ M, FERRAGUD E, REDONDO C, GRAHAM JH, CUBERO J. Chemotactic responses of Xanthomonas with different host ranges. Microorganisms, 2022, 11(1): 43. DOI:10.3390/microorganisms11010043 |
| [54] | ANDRADE MO, Da SILVA JC, SOPRANO AS, SHIMO HM, LEME AFP, BENEDETTI CE. Suppression of Citrus canker disease mediated by flagellin perception. Molecular Plant Pathology, 2023, 24(4): 331-345. DOI:10.1111/mpp.13300 |
| [55] | RABBEE MF, ISLAM N, BAEK KH. Biocontrol of citrus bacterial canker caused by Xanthomonas citri subsp. citri by Bacillus velezensis. Saudi Journal of Biological Sciences, 2022, 29(4): 2363-2371. DOI:10.1016/j.sjbs.2021.12.005 |
| [56] | VILLAMIZAR S, FERRO JA, CAICEDO JC, ALVES LC. Bactericidal effect of entomopathogenic bacterium Pseudomonas entomophila against Xanthomonas citri reduces Citrus canker disease severity. Frontiers in Microbiology, 2020, 11: 1431. DOI:10.3389/fmicb.2020.01431 |
| [57] | MICHAVILA G, ADLER C, de GREGORIO PR, LAMI MJ, CARAM di SANTO MC, ZENOFF AM, de CRISTOBAL RE, VINCENT PA. Pseudomonas protegens CS1 from the lemon phyllosphere as a candidate for Citrus canker biocontrol agent. Plant Biology (Stuttgart, Germany), 2017, 19(4): 608-617. DOI:10.1111/plb.12556 |
| [58] |
DONG YL, TANG QJ, YI TY, XIAO QM. Screening and identification of antagonistic bacteria against Citrus aanker from soil and determination of its control efficacy. Hunan Agricultural Sciences, 2012(9): 77-80.
(in Chinese) 董玉兰, 唐前君, 易图永, 肖启明. 柑橘溃疡病土壤拮抗菌的筛选、鉴定及防效测定. 湖南农业科学, 2012(9): 77-80. DOI:10.3969/j.issn.1006-060X.2012.09.025 |
| [59] | VIEIRA G, PURIĆ J, MORÃO LG, dos SANTOS JA, INFORSATO FJ, SETTE LD, FERREIRA H, SASS DC. Terrestrial and marine Antarctic fungi extracts active against Xanthomonas citri subsp. citri. Letters in Applied Microbiology, 2018, 67(1): 64-71. DOI:10.1111/lam.12890 |
| [60] | VIEIRA G, KHALIL ZG, CAPON RJ, SETTE LD, FERREIRA H, SASS DC. Isolation and agricultural potential of penicillic acid against Citrus canker. Journal of Applied Microbiology, 2022, 132(4): 3081-3088. DOI:10.1111/jam.15413 |
| [61] | XIE MM, ZHANG YC, LIU LP, ZOU YN, WU QS, KUČA K. Mycorrhiza regulates signal substance levels and pathogen defense gene expression to resist Citrus canker. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 2019, 47(4): 1161-1167. DOI:10.15835/nbha47411561 |
| [62] |
YAN ZL, CHEN JP, NONG XX, LI X, LUO HY, WEI LL, RUAN JH, GUAN XY, LU S. Isolation and identification of endophytic fungi from citrus cultivars and their inhibitory activity against Xanthomonas citri subsp. citri causing Citrus canker. Guihaia, 2021, 41(7): 1196-1208.
(in Chinese) 颜桢灵, 陈洁萍, 农小霞, 李鑫, 骆海玉, 韦柳柳, 阮家欢, 关祥媛, 陆莎. 柑橘内生真菌的分离鉴定及其发酵产物对柑橘溃疡病菌的抑制活性. 广西植物, 2021, 41(7): 1196-1208. |
| [63] | ALI AHMAD A, ASKORA A, KAWASAKI T, FUJIE M, YAMADA T. The filamentous phage XacF1 causes loss of virulence in Xanthomonas axonopodis pv. citri, the causative agent of Citrus canker disease. Frontiers in Microbiology, 2014, 5: 321. |
| [64] | AHMAD AA, OGAWA M, KAWASAKI T, FUJIE M, YAMADA T. Characterization of bacteriophages Cp1 and Cp2, the strain-typing agents for Xanthomonas axonopodis pv. citri. Applied and Environmental Microbiology, 2014, 80(1): 77-85. DOI:10.1128/AEM.02310-13 |
| [65] | YOSHIKAWA G, ASKORA A, BLANC-MATHIEU R, KAWASAKI T, LI YZ, NAKANO M, OGATA H, YAMADA T. Xanthomonas citri jumbo phage XacN1 exhibits a wide host range and high complement of tRNA genes. Scientific Reports, 2018, 8: 4486. DOI:10.1038/s41598-018-22239-3 |
| [66] | BALOGH B, CANTEROS BI, STALL RE, JONES JB. Control of Citrus canker and Citrus bacterial spot with bacteriophages. Plant Disease, 2008, 92(7): 1048-1052. DOI:10.1094/PDIS-92-7-1048 |
| [67] | IBRAHIM YE, SALEH AA, AL-SALEH MA. Management of Asiatic Citrus canker under field conditions in Saudi Arabia using bacteriophages and acibenzolar-S-methyl. Plant Disease, 2017, 101(5): 761-765. DOI:10.1094/PDIS-08-16-1213-RE |
| [68] |
XIAO X, DING L, CONG Y, XU XL, QIAO H, HE SL, XU WJ, XU TS. Isolation and identification of a lytic bacteriophage infecting Xanthomonas axonopodis pv. citri. Acta Horticulturae Sinica, 2021, 48(12): 2349-2359.
(in Chinese) 肖逍, 丁良, 丛郁, 徐旭凌, 乔欢, 何四龙, 许文建, 徐天舜. 柑橘溃疡病菌噬菌体的分离鉴定. 园艺学报, 2021, 48(12): 2349-2359. DOI:10.16420/j.issn.0513-353x.2020-0866 |
| [69] | WATTANA-AMORN P, CHAROENWONGSA W, WILLIAMS C, CRUMP MP, APICHAISATAIENCHOTE B. Antibacterial activity of cyclo(L-Pro-L-Tyr) and cyclo(D-Pro-L-Tyr) from Streptomyces sp. strain 22-4 against phytopathogenic bacteria. Natural Product Research, 2016, 30(17): 1980-1983. DOI:10.1080/14786419.2015.1095747 |
| [70] | HABIBOLLAHI F, HOSSEINIPOUR A, LOHRASBI-NEJAD A. Antibacterial activity of theCAP18peptide against Xanthomonas citris sp. citri, the causative agent of Citrus canker, as evaluated by in vitro and in silico studies. Annals of Applied Biology, 2022, 181(1): 93-106. DOI:10.1111/aab.12762 |
| [71] | WANG X, LIANG LQ, SHAO H, YE XX, YANG XB, CHEN XY, SHI Y, ZHANG LH, XU LH, WANG JX. Isolation of the novel strain Bacillus amyloliquefaciens F9 and identification of lipopeptide extract components responsible for activity against Xanthomonas citri subsp. citri. Plants (Basel, Switzerland), 2022, 11(3): 457. |
| [72] |
CHEN L, WANG ZK, HUANG GJ, CAO YQ, XIA YX, YIN Y. Evaluation of Bacillus subtilis strain CQBS03 against Xanthomonas axonopodis pv. citri. Scientia Agricultura Sinica, 2008, 41(8): 2537-2545.
(in Chinese) 陈力, 王中康, 黄冠军, 曹月青, 夏玉先, 殷幼平. 柑橘溃疡病生防菌株CQBS03的鉴定及其培养特性研究. 中国农业科学, 2008, 41(8): 2537-2545. DOI:10.3864/j.issn.0578-1752.2008.08.046 |
| [73] | YANG RH, SHI Q, HUANG TT, YAN YC, LI SZ, FANG Y, LI Y, LIU LL, LIU LY, WANG XZ, PENG YZ, FAN JB, ZOU LF, LIN SJ, CHEN GY. The natural pyrazolotriazine pseudoiodinine from Pseudomonas mosselii 923 inhibits plant bacterial and fungal pathogens. Nature Communications, 2023, 14: 734. DOI:10.1038/s41467-023-36433-z |
| [74] | RAMOS YG, DUIN IM, DA SILVA MRL, J NIOR RPL. Bioactive copper and Bacillus subtilis for the control and resistance induction against Citrus canker in sweet orange [Citrus sinensis (L.) Osbeck] orchard establishment. Scientia Horticulturae, 2022, 303: 111238. DOI:10.1016/j.scienta.2022.111238 |
| [75] |
LAI JH, SONG SL, LIU B. Effects of three endophytic bacteria with the characteristic of controlling Citrus canker on the activity of several defensive enzymes in navel orange. Acta Agriculturae Zhejiangensis, 2020, 32(11): 1994-2000.
(in Chinese) 赖家豪, 宋水林, 刘冰. 三株柑橘溃疡病生防内生细菌对脐橙感染溃疡病后几种防御酶活性的影响. 浙江农业学报, 2020, 32(11): 1994-2000. DOI:10.3969/j.issn.1004-1524.2020.11.09 |
| [76] | RIERA N, WANG H, LI Y, LI JY, PELZ-STELINSKI K, WANG N. Induced systemic resistance against Citrus canker disease by rhizobacteria. Phytopathology, 2018, 108(9): 1038-1045. DOI:10.1094/PHYTO-07-17-0244-R |
| [77] | GE J, LI D, DING J, XIAO X, LIANG Y. Microbial coexistence in the rhizosphere and the promotion of plant stress resistance: a review. Environmental Research, 2023, 222: 115298. DOI:10.1016/j.envres.2023.115298 |
| [78] | MACHADO D, MAISTRENKO OM, ANDREJEV S, KIM Y, BORK P, PATIL KR, PATIL KR. Polarization of microbial communities between competitive and cooperative metabolism. Nature Ecology & Evolution, 2021, 5(2): 195-203. |
| [79] | FREY-KLETT P, BURLINSON P, DEVEAU A, BARRET M, TARKKA M, SARNIGUET A. Bacterial-fungal interactions: hyphens between agricultural, clinical, environmental, and food microbiologists. Microbiology and Molecular Biology Reviews: MMBR, 2011, 75(4): 583-609. DOI:10.1128/MMBR.00020-11 |
| [80] | RAAIJMAKERS JM, MAZZOLA M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annual Review of Phytopathology, 2012, 50: 403-424. DOI:10.1146/annurev-phyto-081211-172908 |
| [81] | BLASEY N, REHRMANN D, RIEBISCH AK, MÜHLEN S. Targeting bacterial pathogenesis by inhibiting virulence-associated Type Ⅲ and Type Ⅳ secretion systems. Frontiers in Cellular and Infection Microbiology, 2023, 12: 1065561. DOI:10.3389/fcimb.2022.1065561 |
| [82] | FENG YM, LONG ZQ, XIANG HM, RAN JN, ZHOU X, YANG S. Research on diffusible signal factor-mediated quorum sensing in Xanthomonas: a mini-review. Molecules, 2023, 28(2): 876. DOI:10.3390/molecules28020876 |
| [83] | YE T, ZHOU T, LI QT, XU XD, FAN XH, ZHANG LH, CHEN SH. Cupriavidus sp. HN-2, a novel quorum quenching bacterial isolate, is a potent biocontrol agent against Xanthomonas campestris pv. campestris. Microorganisms, 2019, 8(1): 45. DOI:10.3390/microorganisms8010045 |
| [84] | TRAN TM, MA ZM, TRIEBL A, NATH S, CHENG YY, GONG BQ, HAN X, WANG JQ, LI JF, WENK MR, TORTA F, MAYOR S, YANG L, MIAO YS. The bacterial quorum sensing signal DSF hijacks Arabidopsis thaliana sterol biosynthesis to suppress plant innate immunity. bioRxiv, 2020, 3(10): e202000720. |
| [85] | YE T, ZHOU T, FAN XH, BHATT P, ZHANG LH, CHEN SH. Acinetobacter lactucae strain QL-1, a novel quorum quenching candidate against bacterial pathogen Xanthomonas campestris pv. campestris. Frontiers in Microbiology, 2019, 10: 2867. DOI:10.3389/fmicb.2019.02867 |
| [86] | FENG GD, LI JL, PAN MK, DENG XQ, CHEN M, YAO Q, ZHU HH. Sphingomonas folii sp. nov., Sphingomonas citri sp. nov. and Sphingomonas citricola sp. nov., isolated from citrus phyllosphere. International Journal of Systematic and Evolutionary Microbiology, 2022. DOI:10.1099/ijsem.0.005492 |
| [87] | GAO H, FENG GD, FENG ZW, YAO Q, LI JL, DENG XQ, LI XR, ZHU HH. Pseudomonas citri sp. nov, a potential novel plant growth promoting bacterium isolated from rhizosphere soil of citrus. Antonie Van Leeuwenhoek, 2023, 116(3): 281-289. DOI:10.1007/s10482-022-01803-y |
| [88] | PAN MK, FENG GD, YAO Q, LI JL, LIU CJ, ZHU HH. Erwinia phyllosphaerae sp. nov., a novel bacterium isolated from phyllosphere of pomelo (Citrus maxima). International Journal of Systematic and Evolutionary Microbiology, 2022, 72(4): 005316. |
 2023, Vol. 63
2023, Vol. 63




