
中国科学院微生物研究所,中国微生物学会
文章信息
- 孔啸鸣, 刘明皓, 黄英. 2023
- KONG Xiaoming, LIU Minghao, HUANG Ying.
- 细菌蛋白酶体及蛋白酶体抑制剂研究进展
- Bacterial proteasomes and proteasome inhibitors
- 微生物学报, 63(6): 2350-2368
- Acta Microbiologica Sinica, 63(6): 2350-2368
-
文章历史
- 收稿日期:2022-09-22
- 网络出版日期:2023-01-04
2. 中国科学院大学生命科学学院, 北京 100049
2. College of Life Sciences, University of Chinese Academy of Sciences, Beijing 100049, China
蛋白酶体广泛存在于真核生物、古菌及细菌(主要是放线菌)中,是细胞内蛋白质受控降解的重要执行者,对维持细胞正常生命活动具有重要作用[1-2]。蛋白酶体是真核生物和古菌生存所必需的,尽管蛋白酶体对细菌生存并非必需,但其在细菌的代谢调控、环境适应和致病性等方面均发挥着重要功能[1-2]。蛋白酶体也是药物研发的重要靶点,其抑制剂已经成为一类重要的临床抗肿瘤药物,并且在风湿免疫类疾病、阿尔茨海默病以及结核病等的治疗中表现出潜在应用价值[3]。开展细菌蛋白酶体相关研究,不仅有助于深入认识细菌生理、生态与进化,也对以细菌蛋白酶体为靶点的抗感染药物开发具有重要价值,并将促进细菌来源蛋白酶体抑制剂的开发和利用[4-6]。本文概括了细菌蛋白酶体与蛋白酶体抑制剂的研究现状,并对未来研究方向进行了展望,期望为细菌蛋白酶体相关研究及其抑制剂的筛选与开发提供参考。
1 细菌蛋白酶体的结构与功能 1.1 细菌蛋白酶体核心颗粒的结构及组装蛋白酶体是一种多亚基蛋白质复合体,所有的蛋白酶体都具有圆柱状的20S核心颗粒(core particle, CP)。CP包含2个α环和2个β环,α环位于圆柱的两端,可与其他调控蛋白相互作用,介导蛋白酶体的门控;β环位于圆柱的中间,降解肽键的活性催化位点位于β环的内表面,以避免发生误降解(图 1A、1B)[1, 7-8]。细菌蛋白酶体CP的α环和β环分别由7个相同的亚基组成,肽酶活性位点位于各β亚基的内侧,因此每个CP共含有14个相同的活性位点。细菌蛋白酶体CP的α亚基和β亚基分别由prcA和prcB基因编码,通过非α-环依赖型途径组装:在CP组装时,游离的α亚基和β亚基首先形成α-β二聚体,然后7个二聚体组成半蛋白酶体(half proteasome, HP),最后半蛋白酶体两两组合形成有活性的CP;少数古菌也按此步骤进行组装(图 1C、1D)[7, 9]。体外实验发现,半蛋白酶体二聚化为CP是组装中耗时最长的步骤,因此抑制该步骤可能是未来蛋白酶体抑制剂研究的新方向[7, 10]。而真核生物和大部分古菌采用α-环依赖型途径组装蛋白酶体CP,即游离的α亚基首先聚集成七聚体环,随后依次招募β亚基结合到α-环上以组成半蛋白酶体,最终形成完整CP (图 1C、1D)[7]。

|
| 图 1 蛋白酶体核心颗粒结构与组装示意图[7-8] Figure 1 The structure and assembly of proteasome particles (CP)[7-8]. The side view (A) and top view (B) of a Mycobacterium tuberculosis proteasome CP[7-8]. The 20S proteasome CP is about 115 Å in diameter and 150 Å in height. Two heptamer rings composed of α-subunits (yellow) are located at both ends of the cylindrical particle, which can interact with other regulatory proteins and mediate the gating of the proteasome. Two heptamer rings composed of β-subunits (blue) are located in the middle of the cylinder, and the peptidase active site is located at the inner surface of the β-rings[7-8]. The modeling is reconstructed based on data from previous work[8]. C: α-ring-independent proteasome assembly. First, free α- and β- subunits form α-β heterodimers. Then, seven heterodimers assemble into a half proteasome (HP). Finally, two HPs are combined into a complete 20S CP. Bacteria and a few archaeal species share this assembly pathway. D: α-ring-dependent proteasome assembly. First, seven α-subunits aggregate into a heptamer α-ring; Then, seven β-subunits are recruited and bounded to the α-ring to form a HP; finally, two HPs are combined into a complete 20S proteasome. Most archaea and eukaryotes use this pathway[7]. |
1.2 细菌蛋白酶体系统的组成和降解蛋白质的基本流程
细菌蛋白酶体功能的正常发挥需要CP、原核泛素样蛋白(prokaryotic ubiquitin-like protein, Pup)以及多种辅助蛋白的共同作用,这些蛋白组成了Pup-蛋白酶体系统(Pup-proteasome system, PPS),相关蛋白的编码基因通常在基因组上也成簇排列(图 2)[1, 11]。

|
| 图 2 细菌蛋白酶体基因簇结构示意图[1, 11] Figure 2 General genetic organizations of Pup-proteasome system (PPS) gene clusters[1, 11]. The genes encoding a bacterial proteasome and related functional proteins are usually clustered in a single operon, including coding genes of proteasome core subunits, Pup, PafA and Dop. Some actinobacteria lack the proteasome core subunits but still hold Pup labeling systems. |
Pup最早发现于结核分枝杆菌(Mycobacterium tuberculosis, Mtb)中,可以标记其他蛋白,使之被蛋白酶体识别降解[11]。Pup长度在60–70个氨基酸残基不等,C端具有保守的二甘氨酸基序和谷氨酰胺/谷氨酸末端[12]。Pup本身缺乏蛋白酶体切割位点,经蛋白酶体释放后可以循环利用[13-14]。Pup也可以多聚化形成类似泛素的长链,但多聚体反而抑制了蛋白降解[15]。底物肽链在蛋白酶体中被分解为3–22个氨基酸残基构成的短肽,经蛋白酶体释放后再迅速被胞质中的肽酶进一步分解成氨基酸[16]。
Pup连接酶PafA可以催化谷氨酸残基和赖氨酸残基的ε-NH2相连,将Pup连接到底物蛋白上[17-18]。Pup脱酰胺/底物去Pup化双功能酶Dop既可将Pup的C端谷氨酰胺残基脱酰胺转化为谷氨酸残基,为其连接到底物上做准备[18];也可去除蛋白质的Pup标签,使其免于被降解[14, 19-20]。PafA和Dop自身也是蛋白酶体的底物,同时还受到酪蛋白水解蛋白酶(caseinolytic protease, Clp)家族蛋白酶的调控[15, 21-22]。
形成环状复合物的ATP酶(ATPase forming a ring-shaped complex, ARC)在分枝杆菌中又被称为分枝杆菌蛋白酶体ATP酶(mycobacterial proteasome ATPase, Mpa),负责识别Pup标记、将底物蛋白解折叠,进而与底物蛋白通过静电作用相结合,并通过2种构象的交替转变介导底物蛋白进入蛋白酶体[23-25]。ARC为同六聚体环,每个亚基包含Pup识别结构域、通道结构域以及ATP酶结构域[24, 26]。此外,ARC的解折叠作用可能也促进Dop酶的去Pup化作用,以保护部分底物蛋白不被降解[19-20]。
一部分放线菌,如棒杆菌属,缺失了蛋白酶体核心亚基,仅保留了Pup化修饰系统。这些放线菌中单独的Pup化修饰系统并不具备降解底物蛋白的功能,但该系统可能调控蛋白降解以外的其他生理活动(图 2)[27]。此外,在放线菌中还有少数其他蛋白如非ATP依赖的Bpa蛋白(又称PafE)[28-29]和AAA家族ATP酶放线菌Cdc48样蛋白(Cdc48-like proteins of actinobacteria, Cpa),也可发挥类似Pup化标记的功能以介导细菌蛋白酶体底物识别[30-31]。
通常,细菌蛋白酶体降解底物蛋白的过程始于底物蛋白的Pup化修饰,即PafA将活化的Pup蛋白连接到底物蛋白上;ARC/Mpa可以结合在CP的任一端的α环上并识别Pup化的底物蛋白,通过ATP供能将底物蛋白解折叠,并转运至CP内部;随后肽链被β亚基分解,完成降解过程[1, 11, 16]。此过程需要Dop活化Pup蛋白为标记做准备,同时Dop也可以选择性地去除Pup标记,从而拯救部分底物蛋白,调控蛋白酶体降解[18, 20](图 3)。

|
| 图 3 细菌蛋白酶体介导的底物降解 Figure 3 Proteolysis mediated by bacterial proteasomes. The substrate protein is labeled with activated Pup through PafA and then recognized by ARC/Mpa. ARC/Mpa unfolds the substrate and subsequently transports it into the 20S proteasome, where it is degraded into peptides. Dop activates Pup by deamidating the glutamine residue at its C-terminal, forming a glutamate residue. Dop can alternatively remove the pup label previously attached to the substrate protein[1]. |
细菌PPS因其具有标记和识别系统而对底物蛋白具有高度选择性。在2株耻垢分枝杆菌蛋白质组研究中,分别鉴定出了41个和52个蛋白酶体底物蛋白[32-33],约占基因组编码蛋白的1%。而在天蓝色链霉菌中鉴定出的蛋白酶体底物蛋白数量达到110个[34-35],大约是分枝杆菌的2倍,这与2类放线菌的基因组大小成正比。上述放线菌蛋白酶体底物蛋白的功能较为多样,主要涉及呼吸作用、脂质和核酸代谢、环境适应、信号传递等。
1.3 细菌蛋白酶体系统的生理功能蛋白酶体在细菌抗逆和应对环境变化中发挥着重要作用,其中包括了自由生活的细菌对温度、射线、营养限制等不利因素的响应,也包括病原细菌对宿主免疫系统与抗菌药物的防御等[4, 36-37]。但在适宜的富营养环境中,缺失PPS对大多数细菌的生存似乎并无显著影响[38-39]。
1.3.1 氮代谢在氮源缺乏条件下,细菌PPS参与调节氮元素的循环利用,以维持细胞生存。蛋白酶体缺陷型分枝杆菌相比于野生型菌株在氮饥饿条件下的存活率大幅下降[39]。正常情况下Mtb在铵盐缺乏时可利用硝酸盐,但是PPS缺陷会造成Mtb亚硝酸盐还原酶缺陷,阻断硝酸盐代谢通路,导致对细胞有毒性的亚硝酸盐积累[40]。此外,耻垢分枝杆菌Pup缺失菌株的氮代谢全局转录调节因子GlnR表达水平大幅下调,表明Pup化系统在氮饥饿响应中也可能独立发挥作用[41-42]。
1.3.2 DNA损伤修复PPS缺陷会导致耻垢分枝杆菌对DNA损伤的耐受性降低。已有多个DNA修复相关蛋白被确认属于蛋白酶体的底物,如介导SOS修复(SOS repair)的主要转录激活子RecA。在DNA损伤因素排除后,耻垢分枝杆菌PPS缺陷株中RecA仍保持较高水平,而野生型的RecA水平会较快下降,表明PPS可能参与了DNA损伤修复完成后相关蛋白的清除[43]。鉴于SOS修复具有高易错性,相关蛋白的及时清除对于维持基因组稳定可能与DNA修复同等重要。
1.3.3 氧化应激分枝杆菌蛋白酶体与其氧化应激反应密切相关。亚硝基化是胞内蛋白的一种氧化性修饰,蛋白质组学分析表明,Mtb中亚硝基化的蛋白质亦可被Pup标记,PPS缺陷会导致Mtb对亚硝酸盐敏感且无法在宿主细胞内生存,暗示PPS可能参与亚硝基化蛋白质的清除[32-33]。巨噬细胞通过合成NO自由基等活性氮中间体对吞噬体中的病原菌起杀伤作用[44]。而PPS缺陷的菌株相较于野生型对NO自由基的耐受性大幅降低,这表明PPS可能参与Mtb对活性氮中间体的防御[44-45]。此外,PPS缺陷的耻垢分枝杆菌对过氧化氢的敏感性反而降低,这可能与其他抗氧化途径的补偿性诱导有关[32]。
目前,蛋白酶体在链霉菌氧化应激反应中的作用尚不清楚。一项研究表明,在天蓝色链霉菌中,PPS的缺陷会导致过氧化物酶积累,使其对氢过氧化枯烯这一氧化剂的敏感度下降[46];但是也有研究认为蛋白酶体或Pup化系统缺陷都导致天蓝色链霉菌对过氧化氢的敏感性增加[34-35]。
1.3.4 耐药性分枝杆菌对叶酸拮抗剂类药物的耐药性依赖于PPS的正常功能。PPS缺陷会导致Mtb和耻垢分枝杆菌失去对磺胺和甲氧苄啶等抗叶酸药物的耐药性[47]。PPS可能通过降解某种阻遏蛋白提高了二氢叶酸还原酶的表达水平,从而促进四氢叶酸的合成,降低抗叶酸药物的效果[36, 47]。PPS对细胞分裂素合成的抑制似乎也导致了分枝杆菌对叶酸拮抗剂类药物的耐药性,表明细胞分裂素可能参与叶酸代谢调控[37]。
1.3.5 离子代谢在放线菌中,PPS或Pup化系统对金属离子稳态调节起到重要作用[35, 48-50]。棒杆菌属放线菌缺乏蛋白酶体CP,但保留了Pup化修饰系统;其Pup敲除株在缺铁时生长受限,表明Pup化系统参与棒杆菌铁稳态调控[39, 51]。棒杆菌铁储存蛋白Ftn和Dps可被Pup标记,进而可能通过ARC介导的构象转变来调节铁离子的储存和释放[51-52]。而在Mtb的arc/mpa或pafA敲除株中,铜感应抑制因子RicR的表达水平相比野生型显著下调,表明PPS也参与细菌铜代谢的调控[48];锌摄取调节因子Zur的调节子中也有多个基因被发现在Mtb的arc/mpa或pafA敲除株中上调[49-50]。近期,本课题组在研究中也发现,PPS缺陷可能削弱链霉菌对一些金属离子的抗性,如抗Cu2+和Fe3+等(结果待发表)。
1.3.6 细菌蛋白酶体的其他生理功能天蓝色链霉菌的蛋白酶体缺陷株表现出孢子形成缺陷,并且出现菌丝体色素沉积减少、气生菌丝发育异常和次级代谢产物合成能力减弱等现象[34-35];玫瑰孢链霉菌的蛋白酶体缺陷导致其达托霉素的合成能力缺失[53]。部分链霉菌的pafA敲除引起孢子形成缺陷和次级代谢能力下降,但这些现象与蛋白酶体无关,暗示Pup化系统也可能独立于蛋白酶体发挥作用[35]。此外,PPS与Mtb的毒素-抗毒素系统的调节有关,多种毒素和抗毒素已被证实可被Pup标记[54-56]。
2 细菌蛋白酶体的系统进化 2.1 细菌蛋白酶体与真核生物、古菌蛋白酶体的比较细菌、真核生物和古菌的蛋白酶体CP结构和催化机理相似,但三者间也存在显著差异[7, 57-59]。细菌蛋白酶体的α环和β环都由同源七聚体组成[60],而真核生物蛋白酶体α环和β环都分别由7个不同的亚基组成,且仅部分β亚基具有蛋白降解活性[7, 61]。古菌则编码多种α亚基或β亚基,在一定条件下其蛋白酶体CP组成会发生变化[2]。此外,真核生物26S蛋白酶体除核心颗粒外还包括19S调控颗粒,后者起到类似于细菌ARC/Mpa等蛋白酶体辅助蛋白的作用[7, 62]。
真核生物和古菌蛋白酶体的组装过程也与细菌蛋白酶体不同(图 1C、1D)。真核生物蛋白酶体的组装为α-环依赖型,并且需要多种伴侣蛋白的作用,才能将14种不同亚基组装成完整的蛋白酶体CP[63-64]。古菌蛋白酶体也主要进行α-环依赖型组装,但未发现其组装需要伴侣蛋白;少数古菌蛋白酶体存在类似于细菌蛋白酶体的非α-环依赖型组装的迹象,可能体现了二者间的进化关联[7, 65-66]。
就门控结构而言,真核生物和细菌蛋白酶体α亚基的N端序列中的疏水氨基酸残基形成了严密的门控,确保蛋白酶体两端封闭;而古菌蛋白酶体的两端存在13 Å的开口,封闭性相对较差[2, 7]。
三域生物的蛋白酶体均通过一定的标记分子识别底物。真核生物的标记分子为泛素,它是一种由76个氨基酸组成、结构规整、能够彼此连接成链的小蛋白质,在真核生物中保守,通过泛素激酶E1、泛素缀合酶E2和泛素连接酶E3组成的泛素化酶系统连接到底物上[67]。古菌蛋白酶体的标记系统包括真核生物泛素激酶E1的同源物Uba1和古菌泛素样小修饰蛋白(ubiquitin-like small archaeal modifier proteins, SAMPs)[2, 59]。相比于泛素和SAMPs,细菌的标记分子Pup的空间结构不规则,以无序卷曲为主,对应的Pup化系统中,不仅酶的种类远少于泛素化系统,其关键酶PafA和Dop与真核生物泛素化酶系统序列也无同源性,且Pup的活化和连接过程不涉及磷酸化[68-70]。
2.2 细菌蛋白酶体的分布与进化蛋白酶体在三域生命中广泛分布,但在细菌域中,目前仅发现放线菌门的大多数类群和硝化螺旋菌门的部分菌株含有蛋白酶体,而大多数已知细菌并不含蛋白酶体[1, 59-60]。已有研究通过分析放线菌主要类群中的模式菌株,认为蛋白酶体在放线菌门中的分布和菌株系统发育高度相关[71-72];这一结论与本课题组近期基于上万株放线菌基因组的统计结果基本相符,但我们发现仍有少数例外,部分亲缘关系较近的属,其蛋白酶体的有无却截然不同,表明蛋白酶体在放线菌中的进化过程可能曾经历了多次基因丢失事件(结果待发表)。棒杆菌属和双歧杆菌属等不含蛋白酶体的放线菌类群仍能正常生存,可能与它们具有其他蛋白酶系统有关[2],并且它们仍保留了Pup化系统(图 2)[51, 71-72]。红球菌菌株NI86/21含有2套不同的蛋白酶体基因簇,GC含量和序列分析表明其中一套可能来自于其他放线菌,说明放线菌门内部的蛋白酶体可能存在水平基因转移现象[60, 73]。
革兰氏阴性细菌硝化螺旋菌门的PPS的序列和组装等与放线菌PPS高度相似[74]。系统发育分析表明,硝化螺旋菌的PPS可能是从放线菌门的酸微菌目水平转移而来[75-76]。异源表达实验表明,Mtb的蛋白酶体可以在亲缘关系较远的大肠杆菌中正常表达与组装,侧面佐证了细菌蛋白酶体发生远缘水平基因转移的可能性[58, 75]。在部分革兰氏阴性细菌中,还发现了另一种潜在的蛋白酶体系统,其基因簇中的pup被细菌泛素(ubiquitin-like protein in bacteria, UBact)的编码基因所取代。UBact与Pup长度相似,具有相同的C端GE/GQ序列,可能也起到标记作用;但是,到目前为止对UBact的研究尚停留在生物信息学层面[74]。
蛋白酶体20S CP与常见于细菌中的HslV (heat shock locus V)蛋白酶可能具有共同的起源(图 4)[77-79]。HslV蛋白酶由2个六聚体环堆叠而成,其肽酶活性需要由ATP酶HslU (heat shock locus U)激活[80]。大多数细菌类群仅含HslUV系统或20S蛋白酶体系统两者之一,但是在多种硝化螺旋菌中发现了同时含有这2种系统的物种,说明2种系统在代谢上并不冲突,多数细菌仅含其中1种系统可能是进化的结果[27, 77]。

|
| 图 4 可能的蛋白酶体核心颗粒亚基进化过程示意图[77-79] Figure 4 A putative evolution process of the subunits of proteasome CPs[77-79]. After the differentiation of α- and β-subunits, an evolutionary branch formed the present archaeal proteasomes. During the process, several homologs of α- and β- subunits were formed, which could participate in proteasome assembly and partially change their structures and functions under certain conditions. The proteasomes of most eukaryotes, such as yeasts, each has seven different α-subunits and seven different β-subunits. During evolution, mammals have formed several tissue-specific homologs of proteasome CP subunits[77-79]. |
2.3 细菌蛋白酶体起源假说
细菌蛋白酶体的起源目前尚无定论。长期以来,主流观点认为放线菌从古菌或真核生物中获得了蛋白酶体(图 4),主要基于以下2点理由:一是除放线菌外几乎所有细菌门都丢失了蛋白酶体这一事件发生概率较低;二是蛋白酶体在通常情况下并非放线菌生存所必需[78, 81],其基因的敲除与突变并不导致致死性变化。
随着对生物早期进化的深入研究,另一种假说被提出,认为放线菌蛋白酶体是原始蛋白酶体垂直遗传和直接进化的产物,与古菌和真核生物蛋白酶体的分化发生于α与β亚基分化之后(图 4)。基于放线菌中发现的磷脂酰肌醇和胆固醇等成分,有研究推测放线菌是新壁总域(neomura, 古菌和真核生物的共同祖先)的姊妹群,那么蛋白酶体可能起源于新壁总域和放线菌门的共同祖先,而后在放线菌、古菌和真核生物中各自独立进化[82],本课题组也倾向于这种假说。
在UBact被发现后,又出现了新的假说,即蛋白酶体起源于现存生物的共同祖先,并且分别被革兰氏阴性细菌、革兰氏阳性细菌和新壁总域继承与发展,细菌中蛋白酶体的大量缺失则源于基因丢失,但是该假说尚需更多证据支持[74, 83]。
3 蛋白酶体抑制剂概述 3.1 蛋白酶体抑制剂的作用机理与分类蛋白酶体抑制剂(proteasome inhibitor, PI)是指能够抑制蛋白酶体肽酶活性的小分子化合物。大多数PIs的结构包括1个短肽或其类似物,以及1个药效基团;短肽部分和蛋白酶体的底物结合口袋相互作用,而药效团结合于β亚基活性位点,阻碍肽链的正常降解。从天然产物中发现了多种新型的白酶体抑制剂,如β-内酯类和环氧酮类[84-85]。根据药效团的结构,可以将PIs分为肽醛、硼酸肽、环氧酮肽等不同类别(表 1)[3, 84, 86]。此外,部分蛋白酶抑制剂也能抑制蛋白酶体的活性,譬如丝氨酸蛋白酶抑制剂3, 4-二氯异香豆素等[87]。
| Class | Feature | Representative compound | Structure of representative compounds | Application |
| Peptide aldehyde | Peptide aldehydes reversibly combine with proteasomes, and can be discharged through multidurg resistance system, thus aren’t suitable for drug development[84, 89] | MG-1321 |  |
Widely used in the laboratories as a standard reagent of proteasome inhibitors[89] |
| Peptide boronate | Peptide boronates have better selectivity and effect than peptide aldehydes. The first proteasome inhibitor put into clinical use belongs to this catagory[84, 90] | Bortezomib1 | 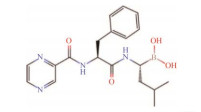 |
Clinically used for the treatment of multiple myeloma and recurrent or refractory mantle cell lymphoma, as a single agent or in combination with other drugs[3, 91] |
| Ixazomib1 |  |
Ixazomib is the first oral proteasome inhibitor antitumor drug used for previously treated multiple myeloma in combination with lenalidomide or dexamethasone[92] | ||
| Peptide vinyl sulfone | Peptide vinyl sulfones can covalently bind to proteasomes irreversibly, have good selectivity and can be easily synthetized[93] | WLL-vs1 |  |
This compound can selectively inhibit Plasmodium proteasome, has low toxicity to mammals, and has the potential to be developed as a new antimalarial drug[94] |
| Peptide α-keto-aldehydes | These compounds are not superior to other classes of proteasome inhibitors in effect and selectivity, thus they are less valued until the development of high-throughput screening[84] | Cbz-Leu-Leu-Tyr-COCHO1 |  |
The compound is a synthetic slow-binding reversible inhibitor against chymotryptic-like activity of proteasome[95] |
| α-keto-amides | NI-010691 |  |
The compound shows antiviral activity in cell experiment, and its EC50 value of SARS-CoV-2 in Caco-2 cell is 1.28 μmol/L. It may have the potential to be developed as a durg for COVID-2019[96] | |
| Indanone peptides | CVT-6341 |  |
The compound can inhibit proteasome trypsin-like activity, and inhibit tumor growth in vitro by blocking NF-κB pathway[97] | |
| β-lactone | Originally found in actinobacterial natural products, including a variety of non-peptide proteasome inhibitors[84, 98] | Lactacystin2 |  |
Cannot bind to proteasome itself, but can be spontaneously transformed into active clasto-lactacystin-β-lactone under neutral conditions[98-99] |
| Marizomib2 |  |
As a candidate antitumor drug, it has entered clinical trial and is the first non-peptide proteasome inhibitor in the process[100-101] | ||
| Peptide epoxyketone | Peptide epoxyketones have high selectivity, mainly bind to the chymotrypsin-like sites and inhibit proteasome activity by reacting with active threonines to form themorpholine rings[102] | Epoxomicin2 |  |
It is a natural product from Actinomycete strain Q996-17. Clinical antitumor drug carfizomib is an efficient and solubility improved product of epoxomicin[102-104] |
| Carfizomib3 |  |
Carfizomib is used in patients with multiple myeloma progressing after treatment with first-line to third-line antitumor drugs. It has better efficacy and tolerance than bortezomib[104-105] | ||
| 1: Synthetic proteasome inhibitors; 2: Natural proteasome inhibitors from actinobacteria; 3: Proteasome inhibitors that modified from actinobacterial biosynthetic products. | ||||
经典PIs作用于蛋白酶体β亚基活性位点,但往往缺乏选择性;针对蛋白酶体系统其他位点的非典型抑制剂可能获得更好的临床效果与更小的不良反应,已有研究发现非典型PIs能与经典PIs协同作用,提高对耐药性肿瘤的药效[88]。
3.2 蛋白酶体抑制剂的发掘得益于检测技术的发展,PI发掘已经进入高通量筛选时代,呈现出筛选文库逐渐扩大、候选化合物多样性逐渐上升、自动化程度逐渐提高的趋势。
3.2.1 基于活性测定的PI筛选筛选PI的基础是测定化合物对蛋白酶体的抑制活性,目前已有荧光法、核磁共振法和基于蛋白定量的方法等多种技术手段。
通过紫外-可见分光光度法测定含发色团的多肽降解时的荧光信号是常用的PI筛选方法。通过设计与蛋白酶体特定亚基具有较高亲和力的多肽,可以筛选特异性PIs,目前已经通过该方法筛选出了一组含S-高苯丙氨酸的肽苄基酰胺类化合物[106-108]。纳米液滴荧光测定法也被用于检测蛋白酶体活性,其灵敏度高,有助于分析微量化合物活性[109]。荧光探针法将荧光基团-PI作为探针,借助荧光测定来比较待测化合物与探针同蛋白酶体靶点的竞争结合能力,从而达到活性测定的效果。这一方法灵敏度高、抗干扰性强,可以测定胞内蛋白酶体抑制活性,有利于筛选具有成药潜力的化合物,但是成本较高;目前这些方法已经被用于检测与验证新型PI,如德兰佐米等的活性[110-111]。
13C核磁共振可以检测底物肽键中标记的13C因肽键降解引起的化学位移变化,该技术耗时短,能适应培养液粗提物等复杂样本,更适用于天然产物筛选[112]。表达融合报告蛋白的细胞系也可用于测定蛋白酶体活性,如泛素-绿色荧光蛋白和泛素-荧光素酶等;当细胞的蛋白酶体功能被抑制时,报告蛋白在细胞中积累,据此可定量测量抑制剂的效力[113-115],但是该方法灵敏度低且易受干扰[108, 116]。
3.2.2 基于计算技术的PI筛选计算机辅助药物设计极大提升了合成PIs的筛选效率。分子对接技术可以根据靶蛋白和配体的结构计算两者之间互作的模式与最低能量的结合状态,并判断互作的强度。目前,利用分子对接技术筛选设计的化合物文库已经成为优化PI结构、寻找新型PIs的重要手段[117-118]。随着分子结构和配体数据库的扩大,应用虚拟筛选发现了一批结构新颖的新型PIs,譬如吡唑骨架非肽化合物G4-1和喹啉-磺胺杂交化合物VR23等[118-121]。
3.2.3 天然PIs的发掘放线菌是细菌中少数具有蛋白酶体的类群,也是PIs的重要来源(表 1)。借助新菌株发掘和沉默基因簇激活等手段从放线菌中不断发现新型PIs,如在Kitasatospora cystarginea中发现新型β-内酯类PI cystargolides,以及在Streptomyces cacaoi中通过优化发酵条件获得具有PI活性的新聚醚类化合物等[122-123]。其中一个典型的例子是最初分离于未鉴定放线菌纲菌株No. Q996-17的环氧酮类化合物环氧酶素,其对真核生物蛋白酶体的抑制活性不足、且理化性质并不利于成药;随后,通过修改环氧酶素的肽链获得了对真核生物蛋白酶体具有强抑制作用和高选择性的化合物YU-101;最后医药公司在该化合物N-末端连接了吗啉环,即得到了目前广泛应用于临床的抗淋巴瘤药物卡非佐米[104]。
放线菌大多含有蛋白酶体,其编码PIs的生物合成基因簇(biosynthetic gene cluster, BGC)中也多含有额外的prcB拷贝,可能发挥着抗性基因的功能,这一特征可作为放线菌中PI类BGCs的筛选标记。例如,热带盐孢菌能够合成PI马利佐米(salinosporamide A, marizomib),其BGC就编码一个对马利佐米耐受性超过PPS簇内β亚基30倍的额外β亚基,以防止马利佐米对自身的毒害作用[124]。本课题组基于此原理建立了一套生信筛选流程,并应用于链霉菌中PI类BGCs的发掘,已从红壤来源的链霉菌分离株中发现了1个可能编码新型环氧酮类PI的BGC,正在开展基因簇激活和鉴定工作(结果待发表)。
此外,微生物的次级代谢物往往具有生态作用,如抑制其他微生物的生存、调节种群间关系或与宿主的关系等。Syringolin A是植物病原菌丁香假单胞菌合成的PI,也是其侵袭植物的一种毒力因子,这是第1个生态作用被阐明的PI;在昆虫和人类病原菌中,已经发现了syringolin A基因簇的同源序列[125-126]。病原菌或共生微生物可能通过抑制宿主蛋白酶体以侵袭宿主或协调种间关系,这意味着共生微生物有潜力成为新型PIs的来源[125, 127]。因此,基于放线菌的生态位和种间关系挖掘新型PIs也是可能的途径[108, 127]。
3.3 蛋白酶体抑制剂的临床应用目前,PIs在临床上主要作为抗肿瘤药物,硼替佐米、卡非佐米等已被批准用于多发性骨髓瘤和套细胞淋巴瘤的单药或联合治疗[128-129]。然而PI类药物也面临严重的耐药性问题,新型PIs的发掘和应用有望解决这一难题[105, 130]。譬如,亚基选择性抑制剂马利佐米能有效抑制对硼替佐米耐药的肿瘤细胞[101, 131];三阴性乳腺癌对硼替佐米和卡非佐米耐药,但是选择性抑制其蛋白酶体β1或β2亚基可恢复对上述药物的敏感性[132-133]。
此外,以免疫蛋白酶体为靶点的特异性抑制剂在治疗自身免疫性疾病和神经系统疾病中具有潜在应用前景。而由于蛋白酶体对某些病原体生存的必要性,蛋白酶体也可能成为新型抗感染药物的靶点,但目前仍缺乏针对细菌蛋白酶体的选择性抑制剂[3]。
4 展望蛋白质被称为生命分子,生物体蛋白质组的调节是生长发育、环境适应等生命过程的关键。蛋白酶体在蛋白质的降解中起到重要作用,因此在生物的代谢网络中具有核心地位[4, 61]。
相比于真核生物蛋白酶体,尽管细菌蛋白酶体(主要是放线菌蛋白酶体)的结构和调控较为简单,但目前仍缺乏对它的深入了解。一方面,迄今细菌蛋白酶体的功能研究主要集中于个别种属,结论缺乏普适性;另一方面,对细菌蛋白酶体功能的认识多停留在现象层面,缺乏深入的机制研究,甚至一些研究结论彼此矛盾[34-35, 46]。未来进一步探索细菌蛋白酶体的功能和相关分子机制,将有助于深入理解细菌代谢调控和环境适应机制,并指导次级代谢新产物和抗菌药物新靶点的发现。
蛋白酶体在进化中高度保守,是生物进化的重要标志物之一[78-79]。但是细菌域中蛋白酶体的实际分布情况和进化轨迹仍不清楚,导致基于蛋白酶体的生命之树仍充满争议[78-79, 82-83]。研究细菌蛋白酶体进化有望揭示蛋白酶体的起源及传播过程,并有助于探索新壁总域的起源以及生命早期进化的脉络[74, 83]。
放线菌是天然PIs的重要来源,随着测序技术的发展,其基因组数据量飞速上升,基因组挖掘将是发现新型PIs的重要手段[134-135]。分枝杆菌属等放线菌类群中包含重要的病原菌,靶向其PPS的抑制剂有望成为新的抗感染药物。因此,针对细菌特有的蛋白酶体系统组分,如Pup化酶系统、ARC/Mpa等,筛选特异性PIs在未来研究中极具前景。
| [1] | JASTRAB JB, DARWIN KH. Bacterial proteasomes. Annual Review of Microbiology, 2015, 69: 109-127. DOI:10.1146/annurev-micro-091014-104201 |
| [2] | DOUGAN DA. Regulated proteolysis in microorganisms. Berlin: Springer, 2013. |
| [3] | SHERMAN DJ, LI J. Proteasome inhibitors: harnessing proteostasis to combat disease. Molecules: Basel, Switzerland, 2020, 25(3): E671. DOI:10.3390/molecules25030671 |
| [4] | MÜLLER AU, WEBER-BAN E. The bacterial proteasome at the core of diverse degradation pathways. Frontiers in Molecular Biosciences, 2019, 6: 23. DOI:10.3389/fmolb.2019.00023 |
| [5] | COLLINS GA, GOLDBERG AL. The logic of the 26S proteasome. Cell, 2017, 169(5): 792-806. DOI:10.1016/j.cell.2017.04.023 |
| [6] | LIN G, LI D, de CARVALHO LP, DENG H, TAO H, VOGT G, WU K, SCHNEIDER J, CHIDAWANYIKA T, WARREN JD, LI H, NATHAN C. Inhibitors selective for mycobacterial versus human proteasomes. Nature, 2009, 461(7264): 621-626. DOI:10.1038/nature08357 |
| [7] | BUDENHOLZER L, CHENG CL, LI Y, HOCHSTRASSER M. Proteasome structure and assembly. Journal of Molecular Biology, 2017, 429(22): 3500-3524. DOI:10.1016/j.jmb.2017.05.027 |
| [8] | DING Z, XU C, SAHU I, WANG Y, FU Z, HUANG M, WONG CCL, GLICKMAN MH, CONG Y. Structural snapshots of 26S proteasome reveal tetraubiquitin-induced conformations. Molecular Cell, 2019, 73(6): 1150-1161.e6. DOI:10.1016/j.molcel.2019.01.018 |
| [9] | ITAGI P, KANTE A, SUPPAHIA A, ROELFS J, DEEDS EJ. Understanding separation of time scales in bacterial proteasome assembly. Biophysical Journal, 2020, 118(3): 517a. |
| [10] | LI D, LI H, WANG T, PAN H, LIN G, LI H. Structural basis for the assembly and gate closure mechanisms of the Mycobacterium tuberculosis 20S proteasome. The EMBO Journal, 2010, 29(12): 2037-2047. |
| [11] | BURNS KE, LIU WT, BOSHOFF HIM, DORRESTEIN PC, BARRY CE 3rd. Proteasomal protein degradation in mycobacteria is dependent upon a prokaryotic ubiquitin-like protein. Journal of Biological Chemistry, 2009, 284(5): 3069-3075. DOI:10.1074/jbc.M808032200 |
| [12] | STRIEBEL F, IMKAMP F, ÖZCELIK D, WEBER-BAN E. Pupylation as a signal for proteasomal degradation in bacteria. Biochimica et Biophysica Acta, 2014, 1843(1): 103-113. DOI:10.1016/j.bbamcr.2013.03.022 |
| [13] | ZERBIB E, SCHLUSSEL S, HECHT N, BAGDADI N, EICHLER J, GUR E. The prokaryotic ubiquitin-like protein presents poor cleavage sites for proteasomal degradation. Cell Reports, 2021, 36(4): 109428. DOI:10.1016/j.celrep.2021.109428 |
| [14] | ELHARAR Y, SCHLUSSEL S, HECHT N, MEIJLER MM, GUR E. The regulatory significance of tag recycling in the mycobacterial pup-proteasome system. The FEBS Journal, 2017, 284(12): 1804-1814. DOI:10.1111/febs.14086109428 |
| [15] | ALHUWAIDER AAH, TRUSCOTT KN, DOUGAN DA. Pupylation of PafA or pup inhibits components of the pup-proteasome system. FEBS Letters, 2018, 592(1): 15-23. DOI:10.1002/1873-3468.12930 |
| [16] | KISSELEV AF, AKOPIAN TN, WOO KM, GOLDBERG AL. The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Journal of Biological Chemistry, 1999, 274(6): 3363-3371. DOI:10.1074/jbc.274.6.3363 |
| [17] | STRIEBEL F, IMKAMP F, SUTTER M, STEINER M, MAMEDOV A, WEBER-BAN E. Bacterial ubiquitin-like modifier pup is deamidated and conjugated to substrates by distinct but homologous enzymes. Nature Structural & Molecular Biology, 2009, 16(6): 647-651. |
| [18] | DELLEY CL, MÜLLER AU, ZIEMSKI M, WEBER-BAN E. Prokaryotic ubiquitin-like protein and its ligase/deligase enyzmes. Journal of Molecular Biology, 2017, 429(22): 3486-3499. DOI:10.1016/j.jmb.2017.04.020 |
| [19] | BURNS KE, CERDA-MAIRA FA, WANG T, LI H, BISHAI WR, DARWIN KH. "Depupylation" of prokaryotic ubiquitin-like protein from mycobacterial proteasome substrates. Molecular Cell, 2010, 39(5): 821-827. DOI:10.1016/j.molcel.2010.07.019 |
| [20] | IMKAMP F, STRIEBEL F, SUTTER M, OZCELIK D, ZIMMERMANN N, SANDER P, WEBER-BAN E. Dop functions as a depupylase in the prokaryotic ubiquitin-like modification pathway. EMBO Reports, 2010, 11(10): 791-797. DOI:10.1038/embor.2010.119 |
| [21] | KORMAN M, SCHLUSSEL S, VISHKAUTZAN M, GUR E. Multiple layers of regulation determine the cellular levels of the pup ligase PafA in Mycobacterium smegmatis. Molecular Microbiology, 2019, 112(2): 620-631. DOI:10.1111/mmi.14278 |
| [22] | HECHT N, BECHER M, KORMAN M, VISHKAUTZAN M, GUR E. Inter- and intramolecular regulation of protein depupylation in Mycobacterium smegmatis. The FEBS Journal, 2020, 287(20): 4389-4400. DOI:10.1111/febs.15245 |
| [23] | DJURANOVIC S, HARTMANN MD, HABECK M, URSINUS A, ZWICKL P, MARTIN J, LUPAS AN, ZETH K. Structure and activity of the N-terminal substrate recognition domains in proteasomal ATPases. Molecular Cell, 2009, 34(5): 580-590. DOI:10.1016/j.molcel.2009.04.030 |
| [24] | WANG T, DARWIN KH, LI H. Binding-induced folding of prokaryotic ubiquitin-like protein on the Mycobacterium proteasomal ATPase targets substrates for degradation. Nature Structural & Molecular Biology, 2010, 17(11): 1352-1357. |
| [25] | KAVALCHUK M, JOMAA A, JOMAA A, MÜLLER AU, WEBER-BAN E. Structural basis of prokaryotic ubiquitin-like protein engagement and translocation by the mycobacterial mpa-proteasome complex. Nature Communications, 2022, 13(1): 276. DOI:10.1038/s41467-021-27787-3 |
| [26] | STRIEBEL F, HUNKELER M, SUMMER H, WEBER-BAN E. The mycobacterial mpa-proteasome unfolds and degrades pupylated substrates by engaging pup's N-terminus. Journal of Healthcare Engineering, 2010, 29(7): 1262-1271. |
| [27] | de MOT R, NAGY I, WALZ J, BAUMEISTER W. Proteasomes and other self-compartmentalizing proteases in prokaryotes. Trends in Microbiology, 1999, 7(2): 88-92. DOI:10.1016/S0966-842X(98)01432-2 |
| [28] | DELLEY CL, LAEDERACH J, ZIEMSKI M, BOLTEN M, BOEHRINGER D, WEBER-BAN E. Bacterial proteasome activator bpa (rv3780) is a novel ring-shaped interactor of the mycobacterial proteasome. PLoS One, 2014, 9(12): e114348. DOI:10.1371/journal.pone.0114348 |
| [29] | JASTRAB JB, WANG T, MURPHY JP, BAI L, HU K, MERKX R, HUANG J, CHATTERJEE C, OVAA H, GYGI SP, LI H, DARWIN KH. An adenosine triphosphate-independent proteasome activator contributes to the virulence of Mycobacterium tuberculosis. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(14): E1763-E1772. |
| [30] | UNCIULEAC MC, SMITH PC, SHUMAN S. Crystal structure and biochemical characterization of a Mycobacterium smegmatis AAA-type nucleoside triphosphatase phosphohydrolase (Msm0858). Journal of Bacteriology, 2016, 198(10): 1521-1533. DOI:10.1128/JB.00905-15 |
| [31] | ZIEMSKI M, JOMAA A, JOMAA A, MAYER D, RUTZ S, GIESE C, VEPRINTSEV D, WEBER-BAN E. Cdc48-like protein of actinobacteria (Cpa) is a novel proteasome interactor in mycobacteria and related organisms. eLife, 2018, 7: e34055. DOI:10.7554/eLife.34055 |
| [32] | WATROUS J, BURNS K, LIU WT, PATEL A, HOOK V, BAFNA V, BARRY 3RD CE, BARK S, DORRESTEIN PC. Expansion of the mycobacterial "PUPylome". Molecular BioSystems, 2010, 6(2): 376-385. DOI:10.1039/B916104J |
| [33] | POULSEN C, AKHTER Y, JEON AH, SCHMITT-ULMS G, MEYER HE, STEFANSKI A, STÜHLER K, WILMANNS M, SONG YH. Proteome-wide identification of mycobacterial pupylation targets. Molecular Systems Biology, 2010, 6: 386. DOI:10.1038/msb.2010.39 |
| [34] | BOUBAKRI H, SEGHEZZI N, DUCHATEAU M, GOMINET M, KOFROŇOVÁ O, BENADA O, MAZODIER P, PERNODET JL. The absence of pupylation (prokaryotic ubiquitin-like protein modification) affects morphological and physiological differentiation in Streptomyces coelicolor. Journal of Bacteriology, 2015, 197(21): 3388-3399. DOI:10.1128/JB.00591-15 |
| [35] | COMPTON CL, FERNANDOPULLE MS, NAGARI RT, SELLO JK. Genetic and proteomic analyses of pupylation in Streptomyces coelicolor. Journal of Bacteriology, 2015, 197(17): 2747-2753. DOI:10.1128/JB.00302-15 |
| [36] | von ROSEN T, KELLER LM, WEBER-BAN E. Survival in hostile conditions: pupylation and the proteasome in actinobacterial stress response pathways. Frontiers in Molecular Biosciences, 2021, 8: 685757. DOI:10.3389/fmolb.2021.685757 |
| [37] | GUZZO MB, LI Q, NGUYEN HV, BOOM WH, NGUYEN L. The pup-proteasome system protects mycobacteria from antimicrobial antifolates. Antimicrobial Agents and Chemotherapy, 2021, 65(4): e01967-e01920. |
| [38] | BECKER SH, DARWIN KH. Bacterial proteasomes: mechanistic and functional insights. Microbiology and Molecular Biology Reviews, 2017, 81(1): 1-20. |
| [39] | KÜBERL A, FRÄNZEL B, EGGELING L, POLEN T, WOLTERS DA, BOTT M. Pupylated proteins in Corynebacterium glutamicum revealed by MudPIT analysis. Proteomics, 2014, 14(12): 1531-1542. DOI:10.1002/pmic.201300531 |
| [40] | BECKER SH, JASTRAB JB, DHABARIA A, CHATON CT, RUSH JS, KOROTKOV KV, UEBERHEIDE B, DARWIN KH. The Mycobacterium tuberculosis pup-proteasome system regulates nitrate metabolism through an essential protein quality control pathway. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(8): 3202-3210. DOI:10.1073/pnas.1819468116 |
| [41] | ELHARAR Y, ROTH Z, HERMELIN I, MOON A, PERETZ G, SHENKERMAN Y, VISHKAUTZAN M, KHALAILA I, GUR E. Survival of mycobacteria depends on proteasome-mediated amino acid recycling under nutrient limitation. The EMBO Journal, 2014, 33(16): 1802-1814. DOI:10.15252/embj.201387076 |
| [42] | FASCELLARO G, PETRERA A, LAI ZW, NANNI P, GROSSMANN J, BURGER S, BINIOSSEK ML, GOMEZ-AULI A, SCHILLING O, IMKAMP F. Comprehensive proteomic analysis of nitrogen-starved Mycobacterium smegmatis δpup reveals the impact of pupylation on nitrogen stress response. Journal of Proteome Research, 2016, 15(8): 2812-2825. DOI:10.1021/acs.jproteome.6b00378 |
| [43] | MÜLLER AU, IMKAMP F, WEBER-BAN E. The mycobacterial LexA/RecA-independent DNA damage response is controlled by PafBC and the pup-proteasome system. Cell Reports, 2018, 23(12): 3551-3564. DOI:10.1016/j.celrep.2018.05.073 |
| [44] | HUANG L, NAZAROVA EV, RUSSELL DG. Mycobacterium tuberculosis: bacterial fitness within the host macrophage. Microbiology Spectrum, 2019, 7(2). DOI:10.1128/microbiolspec.BAI-0001-2019 |
| [45] | DARWIN KH, EHRT S, GUTIERREZ-RAMOS JC, WEICH N, NATHAN CF. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science, 2003, 302(5652): 1963-1966. DOI:10.1126/science.1091176 |
| [46] | de MOT R, SCHOOFS G, NAGY I. Proteome analysis of Streptomyces coelicolor mutants affected in the proteasome system reveals changes in stress-responsive proteins. Archives of Microbiology, 2007, 188(3): 257-271. DOI:10.1007/s00203-007-0243-8 |
| [47] | AMICI M, SAGRATINI D, PETTINARI A, PUCCIARELLI S, ANGELETTI M, ELEUTERI AM. 20S proteasome mediated degradation of DHFR: implications in neurodegenerative disorders. Archives of Biochemistry and Biophysics, 2004, 422(2): 168-174. DOI:10.1016/j.abb.2003.12.014 |
| [48] | SERAFINI A, PISU D, PALÙ G, RODRIGUEZ GM, MANGANELLI R. The ESX-3 secretion system is necessary for iron and zinc homeostasis in Mycobacterium tuberculosis. PLoS One, 2013, 8(10): e78351. DOI:10.1371/journal.pone.0078351 |
| [49] | FESTA RA, JONES MB, BUTLER-WU S, SINSIMER D, GERADS R, BISHAI WR, PETERSON SN, DARWIN KH. A novel copper-responsive regulon in Mycobacterium tuberculosis. Molecular Microbiology, 2011, 79(1): 133-148. DOI:10.1111/j.1365-2958.2010.07431.x |
| [50] | SAMANOVIC MI, DARWIN KH. Game of somes: protein destruction for Mycobacterium tuberculosis pathogenesis. Trends in Microbiology, 2016, 24(1): 26-34. DOI:10.1016/j.tim.2015.10.001 |
| [51] | KÜBERL A, POLEN T, BOTT M. The pupylation machinery is involved in iron homeostasis by targeting the iron storage protein ferritin. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(17): 4806-4811. DOI:10.1073/pnas.1514529113 |
| [52] | ANDREWS SC. The Ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochimica et Biophysica Acta, 2010, 1800(8): 691-705. DOI:10.1016/j.bbagen.2010.05.010 |
| [53] | XU WF, FANG JL, BU QT, LYU ZY, ZHU CY, SUN CF, ZHAO QW, LI YQ. A novel strategy of gene screen based on multi-omics in Streptomyces roseosporus. Applied Microbiology and Biotechnology, 2022, 106(8): 3103-3112. DOI:10.1007/s00253-022-11904-3 |
| [54] | FESTA RA, MCALLISTER F, PEARCE MJ, MINTSERIS J, BURNS KE, GYGI SP, DARWIN KH. Prokaryotic ubiquitin-like protein (pup) proteome of Mycobacterium tuberculosis corrected. PLoS One, 2010, 5(1): e8589. DOI:10.1371/journal.pone.0008589 |
| [55] | CHI X, CHANG Y, LI M, LIN J, LIU Y, LI C, TANG S, ZHANG J. Biochemical characterization of mt-PemIK, a novel toxin-antitoxin system in Mycobacterium tuberculosis. FEBS Letters, 2018, 592(24): 4039-4050. DOI:10.1002/1873-3468.13280 |
| [56] | BORDES P, GENEVAUX P. Control of toxin-antitoxin systems by proteases in Mycobacterium tuberculosis. Frontiers in Molecular Biosciences, 2021, 8: 691399. DOI:10.3389/fmolb.2021.691399 |
| [57] | ORLOWSKI M, WILK S. Catalytic activities of the 20S proteasome, a multicatalytic proteinase complex. Archives of Biochemistry and Biophysics, 2000, 383(1): 1-16. DOI:10.1006/abbi.2000.2036 |
| [58] | MARQUES AJ, PALANIMURUGAN R, MATIAS AC, RAMOS PC, DOHMEN RJ. Catalytic mechanism and assembly of the proteasome. Chemical Reviews, 2009, 109(4): 1509-1536. DOI:10.1021/cr8004857 |
| [59] | MAUPIN-FURLOW J. Proteasomes and protein conjugation across domains of life. Nature Reviews Microbiology, 2011, 10(2): 100-111. |
| [60] | de MOT R, NAGY I, BAUMEISTER W. A self-compartmentalizing protease in Rhodococcus: the 20S proteasome. Antonie van Leeuwenhoek International Journal of General and Molecular Microbiology, 1998, 74(1-3): 83-87. |
| [61] | BARD JAM, GOODALL EA, GREENE ER, JONSSON E, DONG KC, MARTIN A. Structure and function of the 26S proteasome. Annual Review of Biochemistry, 2018, 87: 697-724. DOI:10.1146/annurev-biochem-062917-011931 |
| [62] | FINLEY D, CHEN X, WALTERS KJ. Gates, channels, and switches: elements of the proteasome machine. Trends in Biochemical Sciences, 2016, 41(1): 77-93. DOI:10.1016/j.tibs.2015.10.009 |
| [63] | MARSHALL RS, GEMPERLINE DC, MCLOUGHLIN F, Book AJ, HOFMANN K, VIERSTRA RD. An evolutionarily distinct chaperone promotes 20S proteasome α-ring assembly in plants. Journal of Cell Science, 2020, 133(21): jcs249862. DOI:10.1242/jcs.249862 |
| [64] | MORRIS EP, da FONSECA PCA. How to build a proteasome. Nature Structural & Molecular Biology, 2021, 28(5): 409-410. |
| [65] | MAUPIN-FURLOW JA, WILSON HL, KACZOWKA SJ, OU MS. Proteasomes in the archaea: from structure to function. Frontiers in Bioscience, 2000, 5: D837-D865. |
| [66] | PANFAIR D, RAMAMURTHY A, KUSMIERCZYK AR. Alpha-ring independent assembly of the 20S proteasome. Scientific Reports, 2015, 5: 13130. DOI:10.1038/srep13130 |
| [67] | NEUTZNER M, NEUTZNER A. Enzymes of ubiquitination and deubiquitination. Essays in Biochemistry, 2012, 52: 37-50. DOI:10.1042/bse0520037 |
| [68] | HUMBARD MA, MIRANDA HV, LIM JM, KRAUSE DJ, PRITZ JR, ZHOU G, CHEN S, WELLS L, MAUPIN-FURLOW JA. Ubiquitin-like small archaeal modifier proteins (SAMPs) in Haloferax volcanii. Nature, 2010, 463(7277): 54-60. DOI:10.1038/nature08659 |
| [69] | MIRANDA HV, NEMBHARD N, SU D, HEPOWIT N, KRAUSE DJ, PRITZ JR, PHILLIPS C, SÖLL D, MAUPIN-FURLOW JA. E1- and ubiquitin-like proteins provide a direct link between protein conjugation and sulfur transfer in archaea. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(11): 4417-4422. DOI:10.1073/pnas.1018151108 |
| [70] | BARANDUN J, DAMBERGER FF, DELLEY CL, LAEDERACH J, ALLAIN FH, WEBER-BAN E, WEBER-BAN E. Prokaryotic ubiquitin-like protein remains intrinsically disordered when covalently attached to proteasomal target proteins. BMC Structural Biology, 2017, 17(1): 1. |
| [71] | BaRANDUN J, DELLEY CL, WEBER-BAN E. The pupylation pathway and its role in mycobacteria. BMC Biology, 2012, 10: 95. DOI:10.1186/1741-7007-10-95 |
| [72] | DARWIN KH. Prokaryotic ubiquitin-like protein (pup), proteasomes and pathogenesis. Nature Reviews Microbiology, 2009, 7(7): 485-491. DOI:10.1038/nrmicro2148 |
| [73] | TAMURA T, NAGY I, LUPAS A, LOTTSPEICH F, CEJKA Z, SCHOOFS G, TANAKA K, de MOT R, BAUMEISTER W. The first characterization of a eubacterial proteasome: the 20S complex of Rhodococcus. AJNR American Journal of Neuroradiology, 1995, 5(7): 766-774. |
| [74] | LEHMANN G, UDASIN RG, LIVNEH I, CIECHANOVER A. Identification of UBact, a ubiquitin-like protein, along with other homologous components of a conjugation system and the proteasome in different Gram-negative bacteria. Biochemical and Biophysical Research Communications, 2017, 483(3): 946-950. DOI:10.1016/j.bbrc.2017.01.037 |
| [75] | de MOT R. Actinomycete-like proteasomes in a Gram-negative bacterium. Trends in Microbiology, 2007, 15(8): 335-338. DOI:10.1016/j.tim.2007.06.002 |
| [76] | IYER LM, BURROUGHS AM, ARAVIND L. Unraveling the biochemistry and provenance of pupylation: a prokaryotic analog of ubiquitination. Biology Direct, 2008, 3: 45. DOI:10.1186/1745-6150-3-45 |
| [77] | GILLE C, GOEDE A, SCHLÖETELBURG C, PREISSNER R, KLOETZEL PM, GÖBEL UB, FRÖMMEL C. A comprehensive view on proteasomal sequences: implications for the evolution of the proteasome. Journal of Molecular Biology, 2003, 326(5): 1437-1448. DOI:10.1016/S0022-2836(02)01470-5 |
| [78] | VOLKER C, LUPAS AN. Molecular evolution of proteasomes[M]// Current Topics in Microbiology and Immunology. Berlin, Heidelberg: Springer Berlin Heidelberg, 2002: 1-22. |
| [79] | HUGHES AL. Evolution of the proteasome components. Immunogenetics, 1997, 46(2): 82-92. DOI:10.1007/s002510050245 |
| [80] | RASHID Y, KAMRAN AZIM M, SAIFY ZS, KHAN KM, KHAN R. Small molecule activators of proteasome-related HslV peptidase. Bioorganic & Medicinal Chemistry Letters, 2012, 22(19): 6089-6094. |
| [81] | LUPAS A, ZÜHL F, TAMURA T, WOLF S, NAGY I, de MOT R, BAUMEISTER W. Eubacterial proteasomes. Plants: Basel, Switzerland, 1997, 24(1/2): 125-131. |
| [82] | CAVALIER-SMITH T. The neomuran revolution and phagotrophic origin of eukaryotes and cilia in the light of intracellular coevolution and a revised tree of life. Cold Spring Harbor Perspectives in Biology, 2014, 6(9): a016006. DOI:10.1101/cshperspect.a016006 |
| [83] | CAVALIER-SMITH T, CHAO EE. Multidomain ribosomal protein trees and the planctobacterial origin of neomura (eukaryotes, archaebacteria). Protoplasma, 2020, 257(3): 621-753. DOI:10.1007/s00709-019-01442-7 |
| [84] | KISSELEV AF, GOLDBERG AL. Proteasome inhibitors: from research tools to drug candidates. Chemistry & Biology, 2001, 8(8): 739-758. |
| [85] | KOGUCHI Y, KOHNO J, NISHIO M, TAKAHASHI K, OKUDA T, OHNUKI T, KOMATSUBARA S. TMC-95A, B, C, and D, novel proteasome inhibitors produced by Apiospora montagnei Sacc. TC 1093. Taxonomy, production, isolation, and biological activities. Journal of Wrist Surgery, 2000, 53(2): 105-109. |
| [86] | PAUTASSO C, TROIA R, GENUARDI M, PALUMBO A. Pharmacophore modeling technique applied for the discovery of proteasome inhibitors. Expert Opinion on Drug Discovery, 2014, 9(8): 931-943. DOI:10.1517/17460441.2014.923838 |
| [87] | ORLOWSKI M, CARDOZO C, ELEUTERI AM, KOHANSKI R, KAM CM, POWERS JC. Reactions of [14C]-3, 4-dichloroisocoumarin with subunits of pituitary and spleen multicatalytic proteinase complexes (proteasomes). Biochemistry, 1997, 36(45): 13946-13953. DOI:10.1021/bi970666e |
| [88] | MULI CS, TIAN W, TRADER DJ. Small-molecule inhibitors of the proteasome's regulatory particle. Chembiochem, 2019, 20(14): 1739-1753. |
| [89] | TSUBUKI S, SAITO Y, TOMIOKA M, ITO H, KAWASHIMA S. Differential inhibition of calpain and proteasome activities by peptidyl aldehydes of di-leucine and tri-leucine. The Journal of Biochemistry, 1996, 119(3): 572-576. DOI:10.1093/oxfordjournals.jbchem.a021280 |
| [90] | ADAMS J, BEHNKE M, CHEN S, CRUICKSHANK AA, DICK LR, GRENIER L, KLUNDER JM, MA YT, PLAMONDON L, STEIN RL. Potent and selective inhibitors of the proteasome: dipeptidyl boronic acids. Bioorganic & Medicinal Chemistry Letters, 1998, 8(4): 333-338. |
| [91] | RICHARDSON PG, SONNEVELD P, SCHUSTER MW, IRWIN D, STADTMAUER EA, FACON T, HAROUSSEAU JL, BEN-YEHUDA D, LONIAL S, GOLDSCHMIDT H, REECE D, SAN-MIGUEL JF, BLADÉ J, BOCCADORO M, CAVENAGH J, DALTON WS, BORAL AL, ESSELTINE DL, PORTER JB, SCHENKEIN D, et al. Assessment of proteasome inhibition for extending remissions (APEX) investigators. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. Haematologica, 2005, 352(24): 2487-2498. |
| [92] | MOREAU P, MASSZI T, GRZASKO N, BAHLIS NJ, HANSSON M, POUR L, SANDHU I, GANLY P, BAKER BW, JACKSON SR, STOPPA AM, SIMPSON DR, GIMSING P, PALUMBO A, GARDERET L, CAVO M, KUMAR S, TOUZEAU C, BUADI FK, LAUBACH JP, et al. TOURMALINE-MM1 Study Group. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. The New England Journal of Medicine, 2016, 374(17): 1621-1634. DOI:10.1056/NEJMoa1516282 |
| [93] | BOGYO M, MCMASTER JS, GACZYNSKA M, TORTORELLA D, GOLDBERG AL, PLOEGH H. Covalent modification of the active site threonine of proteasomal beta subunits and the Escherichia coli homolog HslV by a new class of inhibitors. Proceedings of the National Academy of Sciences of the United States of America, 1997, 94(13): 6629-6634. DOI:10.1073/pnas.94.13.6629 |
| [94] | LI H, O'DONOGHUE AJ, van der LINDEN WA, XIE SC, YOO E, FOE IT, TILLEY L, CRAIK CS, da FONSECA PC, BOGYO M. Structure- and function-based design of Plasmodium-selective proteasome inhibitors. Nature, 2016, 530(7589): 233-236. DOI:10.1038/nature16936 |
| [95] | LYNAS JF, HARRIOTT P, HEALY A, MCKERVEY MA, WALKER B. Inhibitors of the chymotrypsin-like activity of proteasome based on di- and tri-peptidyl alpha-keto aldehydes (glyoxals). Bioorganic & Medicinal Chemistry Letters, 1998, 8(4): 373-378. |
| [96] | WANG J, LIANG B, CHEN Y, FUK-WOO CHAN J, YUAN S, YE H, NIE L, ZHOU J, WU Y, WU M, HUANG LS, AN J, WARSHEL A, YUEN KY, CIECHANOVER A, HUANG Z, XU Y. A new class of α-ketoamide derivatives with potent anticancer and anti-SARS-CoV-2 activities. Eur J Med Chem, 2021, 215: 113267. DOI:10.1016/j.ejmech.2021.113267 |
| [97] | LUM RT, KERWAR SS, MEYER SM, NELSON MG, SCHOW SR, SHIFFMAN D, WICK MM, JOLY A. A new structural class of proteasome inhibitors that prevent NF-kappa B activation. Biochemical Pharmacology, 1998, 55(9): 1391-1397. DOI:10.1016/S0006-2952(97)00655-2 |
| [98] | FENTEANY G, STANDAERT RF, LANE WS, CHOI S, COREY EJ, SCHREIBER SL. Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Analytical Cellular Pathology: Amsterdam, 1995, 268(5211): 726-731. |
| [99] | DICK LR, CRUIKSHANK AA, DESTREE AT, GRENIER L, MCCORMACK TA, MELANDRI FD, NUNES SL, PALOMBELLA VJ, PARENT LA, PLAMONDON L, STEIN RL. Mechanistic studies on the inactivation of the proteasome by lactacystin in cultured cells. The Journal of Biological Chemistry, 1997, 272(1): 182-188. DOI:10.1074/jbc.272.1.182 |
| [100] | FELING RH, BUCHANAN GO, MINCER TJ, KAUFFMAN CA, JENSEN PR, FENICAL W. Salinosporamide A: a highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus salinospora. Angewandte Chemie: International Ed in English, 2003, 42(3): 355-357. DOI:10.1002/anie.200390115 |
| [101] | SPENCER A, HARRISON S, ZONDER J, BADROS A, LAUBACH J, BERGIN K, KHOT A, ZIMMERMAN T, CHAUHAN D, LEVIN N, MACLAREN A, REICH SD, TRIKHA M, RICHARDSON P. A phase 1 clinical trial evaluating marizomib, pomalidomide and low-dose dexamethasone in relapsed and refractory multiple myeloma (NPI-0052-107): final study results. British Journal of Haematology, 2018, 180(1): 41-51. DOI:10.1111/bjh.14987 |
| [102] | GROLL M, KIM KB, KAIRIES N, HUBER R, CREWS CM. Crystal structure of epoxomicin: 20S proteasome reveals a molecular basis for selectivity of α′, β′-epoxyketone proteasome inhibitors. Journal of the American Chemical Society, 2000, 122(6): 1237-1238. DOI:10.1021/ja993588m |
| [103] | MENG L, MOHAN R, KWOK BH, ELOFSSON M, SIN N, CREWS CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proceedings of the National Academy of Sciences of the United States of America, 1999, 96(18): 10403-10408. DOI:10.1073/pnas.96.18.10403 |
| [104] | KIM KB, CREWS CM. From epoxomicin to carfilzomib: chemistry, biology, and medical outcomes. Natural Product Reports, 2013, 30(5): 600-604. DOI:10.1039/c3np20126k |
| [105] | MANASANCH EE, ORLOWSKI RZ. Proteasome inhibitors in cancer therapy. Nature Reviews Clinical Oncology, 2017, 14(7): 417-433. DOI:10.1038/nrclinonc.2016.206 |
| [106] | KISSELEV AF, GOLDBERG AL. Monitoring activity and inhibition of 26S proteasomes with fluorogenic peptide substrates. Methods in Enzymology, 2005, 398: 364-378. |
| [107] | BLACKBURN C, GIGSTAD KM, HALES P, GARCIA K, JONES M, BRUZZESE FJ, BARRETT C, LIU JX, SOUCY TA, SAPPAL DS, BUMP N, OLHAVA EJ, FLEMING P, DICK LR, TSU C, SINTCHAK MD, BLANK JL. Characterization of a new series of non-covalent proteasome inhibitors with exquisite potency and selectivity for the 20S beta 5-subunit. The Biochemical Journal, 2010, 430(3): 461-476. DOI:10.1042/BJ20100383 |
| [108] | STEIN ML, GROLL M. Applied techniques for mining natural proteasome inhibitors. Biochimica et Biophysica Acta, 2014, 1843(1): 26-38. DOI:10.1016/j.bbamcr.2013.01.017 |
| [109] | GÖTZE S, SABOROWSKI R. Nanodrop fluorometry adopted for microassays of proteasomal enzyme activities. Analytical Biochemistry, 2011, 413(2): 203-205. DOI:10.1016/j.ab.2011.02.023 |
| [110] | BERKERS CR, van LEEUWEN FW, GROOTHUIS TA, PEPERZAK V, van TILBURG EW, BORST J, NEEFJES JJ, OVAA H. Profiling proteasome activity in tissue with fluorescent probes. Molecular Pharmaceutics, 2007, 4(5): 739-748. DOI:10.1021/mp0700256 |
| [111] | BERKERS CR, LEESTEMAKER Y, SCHUURMAN KG, RUGGERI B, JONES-BOLIN S, WILLIAMS M, OVAA H. Probing the specificity and activity profiles of the proteasome inhibitors bortezomib and delanzomib. Molecular Pharmaceutics, 2012, 9(5): 1126-1135. DOI:10.1021/mp2004143 |
| [112] | STEIN ML, BECK P, KAISER M, DUDLER R, BECKER CF, GROLL M. One-shot NMR analysis of microbial secretions identifies highly potent proteasome inhibitor. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(45): 18367-18371. DOI:10.1073/pnas.1211423109 |
| [113] | DANTUMA NP, LINDSTEN K, GLAS R, JELLNE M, MASUCCI MG. Short-lived green fluorescent proteins for quantifying ubiquitin/proteasome-dependent proteolysis in living cells. Nature Biotechnology, 2000, 18(5): 538-543. DOI:10.1038/75406 |
| [114] | AUSSEIL F, SAMSON A, AUSSAGUES Y, VANDENBERGHE I, CREANCIER L, POUNY I, KRUCZYNSKI A, MASSIOT G, BAILLY C. High-throughput bioluminescence screening of ubiquitin-proteasome pathway inhibitors from chemical and natural sources. Journal of Biomolecular Screening, 2007, 12(1): 106-116. DOI:10.1177/1087057106296494 |
| [115] | LONG C, BECK J, CANTAGREL F, MARCOURT L, VENDIER L, DAVID B, PLISSON F, DERGUINI F, VANDENBERGHE I, AUSSAGUES Y, AUSSEIL F, LAVAUD C, SAUTEL F, MASSIOT G. Proteasome inhibitors from Neoboutonia melleri. Journal of Natural Products, 2012, 75(1): 34-47. DOI:10.1021/np200441h |
| [116] | MEINERS S, HEYKEN D, WELLER A, LUDWIG A, STANGL K, KLOETZEL PM, KRÜGER E. Inhibition of proteasome activity induces concerted expression of proteasome genes and de novo formation of mammalian proteasomes. The Journal of Biological Chemistry, 2003, 278(24): 21517-21525. DOI:10.1074/jbc.M301032200 |
| [117] | HUANG SY, ZOU X. Advances and challenges in protein-ligand docking. International Journal of Molecular Sciences, 2010, 11(8): 3016-3034. DOI:10.3390/ijms11083016 |
| [118] | GUEDES RA, SERRA P, SALVADOR JA, GUEDES RC. Computational approaches for the discovery of human proteasome inhibitors: an overview. Molecules: Basel, Switzerland, 2016, 21(7): E927. DOI:10.3390/molecules21070927 |
| [119] | BASSE N, MONTES M, MARÉCHAL X, QIN LX, Bouvier-Durand M, Genin E, Vidal J, Villoutreix BO, Reboud-Ravaux M. Novel organic proteasome inhibitors identified by virtual and in vitro screening. Journal of Medicinal Chemistry, 2010, 53(1): 509-513. DOI:10.1021/jm9011092 |
| [120] | MILLER Z, KIM KS, LEE DM, KASAM V, BAEK SE, LEE KH, ZHANG YY, AO L, CARMONY K, LEE NR, ZHOU S, ZHAO QQ, JANG Y, JEONG HY, ZHAN CG, LEE W, KIM DE, KIM KB. Proteasome inhibitors with pyrazole scaffolds from structure-based virtual screening. Journal of Medicinal Chemistry, 2015, 58(4): 2036-2041. DOI:10.1021/jm501344n |
| [121] | PUNDIR S, VU HY, SOLOMON VR, MCCLURE R, LEE H. VR23: a quinoline-sulfonyl hybrid proteasome inhibitor that selectively kills cancer via cyclin E-mediated centrosome amplification. Cancer Research, 2015, 75(19): 4164-4175. DOI:10.1158/0008-5472.CAN-14-3370 |
| [122] | GILL KA, BERRUÉ F, ARENS JC, CARR G, KERR RG. Cystargolides, 20S proteasome inhibitors isolated from Kitasatospora cystarginea. Journal of Natural Products, 2015, 78(4): 822-826. DOI:10.1021/np501060k |
| [123] | GEZER E, ÜNER G, KÜÇÜKSOLAK M, KURT MÜ, DOĞAN G, KIRMIZIBAYRAK PB, BEDIR E. Undescribed polyether ionophores from Streptomyces cacaoi and their antibacterial and antiproliferative activities. Phytochemistry, 2022, 195: 113038. DOI:10.1016/j.phytochem.2021.113038 |
| [124] | KALE AJ, MCGLINCHEY RP, LECHNER A, MOORE BS. Bacterial self-resistance to the natural proteasome inhibitor salinosporamide A. ACS Chemical Biology, 2011, 6(11): 1257-1264. DOI:10.1021/cb2002544 |
| [125] | GROLL M, SCHELLENBERG B, BACHMANN AS, ARCHER CR, HUBER R, POWELL TK, LINDOW S, KAISER M, DUDLER R. A plant pathogen virulence factor inhibits the eukaryotic proteasome by a novel mechanism. Nature, 2008, 452(7188): 755-758. DOI:10.1038/nature06782 |
| [126] | XIN XF, KVITKO B, HE SY. Pseudomonas syringae: what it takes to be a pathogen. Nature Reviews Microbiology, 2018, 16(5): 316-328. DOI:10.1038/nrmicro.2018.17 |
| [127] | van BERGEIJK DA, TERLOUW BR, MEDEMA MH, van WEZEL GP. Ecology and genomics of actinobacteria: new concepts for natural product discovery. Nature Reviews Microbiology, 2020, 18(10): 546-558. DOI:10.1038/s41579-020-0379-y |
| [128] | WANG X, MEUL T, MEINERS S. Exploring the proteasome system: a novel concept of proteasome inhibition and regulation. Pharmacology & Therapeutics, 2020, 211: 107526. |
| [129] | ORLOWSKI RZ, KUHN DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clinical Cancer Research, 2008, 14(6): 1649-1657. DOI:10.1158/1078-0432.CCR-07-2218 |
| [130] | NARAYANAN S, CAI CY, ASSARAF YG, GUO HQ, CUI Q, WEI L, HUANG JJ, ASHBY CR, CHEN ZS. Targeting the ubiquitin-proteasome pathway to overcome anti-cancer drug resistance. Drug Resistance Updates, 2020, 48: 100663. DOI:10.1016/j.drup.2019.100663 |
| [131] | RUIZ S, KRUPNIK Y, KEATING M, CHANDRA J, PALLADINO M, MCCONKEY D. The proteasome inhibitor NPI-0052 is a more effective inducer of apoptosis than bortezomib in lymphocytes from patients with chronic lymphocytic leukemia. Molecular Cancer Therapeutics, 2006, 5(7): 1836-1843. DOI:10.1158/1535-7163.MCT-06-0066 |
| [132] | WEYBURNE ES, WILKINS OM, SHA Z, WILLIAMS DA, PLETNEV AA, DE BRUIN G, OVERKLEEFT HS, GOLDBERG AL, COLE MD, KISSELEV AF. Inhibition of the proteasome β2 site sensitizes triple-negative breast cancer cells to β5 inhibitors and suppresses Nrf1 activation. Cell Chemical Biology, 2017, 24(2): 218-230. DOI:10.1016/j.chembiol.2016.12.016 |
| [133] | ROLFE M. The holy grail: solid tumor efficacy by proteasome inhibition. Cell Chemical Biology, 2017, 24(2): 125-126. DOI:10.1016/j.chembiol.2017.01.007 |
| [134] | LEE N, HWANG S, KIM J, CHO S, PALSSON B, CHO BK. Mini review: genome mining approaches for the identification of secondary metabolite biosynthetic gene clusters in Streptomyces. Computational and Structural Biotechnology Journal, 2020, 18: 1548-1556. DOI:10.1016/j.csbj.2020.06.024 |
| [135] | JOSE PA, MAHARSHI A, JHA B. Actinobacteria in natural products research: progress and prospects. Microbiological Research, 2021, 246: 126708. DOI:10.1016/j.micres.2021.126708 |
 2023, Vol. 63
2023, Vol. 63




