中国科学院微生物研究所,中国微生物学会
文章信息
- 施淑怡, 崔海滨, 冀星宇, 申素霞, 张燕, 陈芳. 2023
- SHI Shuyi, CUI Haibin, JI Xingyu, SHEN Suxia, ZHANG Yan, CHEN Fang.
- 番茄红素调控肠道菌群对健康影响的研究进展
- Health-enhancing effect of lycopene based on the regulation of intestinal microbiota
- 微生物学报, 63(7): 2563-2572
- Acta Microbiologica Sinica, 63(7): 2563-2572
-
文章历史
- 收稿日期:2022-10-29
- 网络出版日期:2023-02-08
2. 国家果蔬加工工程技术研究中心, 北京 100083;
3. 中粮屯河番茄有限公司, 新疆 乌鲁木齐 830099
2. National Engineering Research Center for Fruits and Vegetables Processing, Beijing 100083, China;
3. COFCO Tunhe Tomato Co., Ltd., Urumqi 830099, Xinjiang, China
我国是番茄种植大国。2020年我国番茄总产量高达6 598万t,约占世界番茄总产量的35%;同时,我国的番茄加工业也发展为食品工业的重要支撑,2020年我国的番茄酱出口总量达85.65万t,总额为6.8亿美元,仅次于番茄加工产品出口大国意大利(数据来源:FAO)。番茄红素(lycopene)是番茄中含量最高(约14.6 mg/100 g)的抗氧化成分[1],具有多种生物学功能,包括抗炎,抗癌,预防糖尿病,保护心血管、肝脏和神经系统等[2]。《中国居民膳食营养素参考摄入量》推荐成人摄入番茄红素的特定建议值为18 mg/d,而番茄是人体获取番茄红素的重要来源。
研究证实,肠道微生物与健康紧密相关,而大肠是微生物的主要寄居地[3]。由于肠道的厌氧环境,大肠中主要的优势菌种包括厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)、变形菌门(Proteobacteria)等菌门中的厌氧菌属、种[4],这些微生物能够直接或间接地促进人体代谢、抵御病原体等以实现对疾病的干预[5]。研究表明番茄红素具有独特的不饱和长链分子结构,可以有效猝灭单态氧和清除自由基,减轻肠道氧化损伤,可能对维持肠道内环境稳定和肠道菌群健康有重要意义[6]。但番茄红素如何调控肠道菌群?对如何对健康影响?前人并未详细的综述与总结。因此,本文在介绍番茄红素的理化性质及其消化、吸收、代谢的基础上,着眼于其对肠道菌群的调控,进而对疾病的干预作用,为未来番茄营养探索及产品开发提供思路和方向。
1 番茄红素的理化性质番茄红素的分子式为C40H56,分子量为536.85 g/mol[7],是一种不饱和、无环的类胡萝卜素,不溶于水、乙醇、甲醇,能溶于四氢呋喃、氯仿、己烷等有机溶剂,在pH 3.5–4.5范围内稳定性最好[8]。番茄红素具有11个线性共轭双键、2个非共轭双键和8个以异戊二烯为单元组成的四萜烯,这赋予其形成多种几何异构体的可能性[9]。
番茄红素存在顺式和反式异构体,在原料番茄中94%–96%的番茄红素为全反式异构体[10],而在人体内,血清和组织中检测出番茄红素顺式异构体占总量的50%以上[11],其中5-顺式异构体是最稳定的异构体[12],表明番茄红素在消化、吸收、代谢过程中完成了异构化。通过对番茄进行热加工和非热加工(酸、辐照、食品机械加工、光、热等)也能使番茄红素发生多种顺式异构化[13]。将番茄汁、番茄酱等在120 ℃加热1 h,顺式番茄红素的比例可以由加热前的4.6%–9.2%提高至27.4%–33.4%;将番茄浆分别在120 ℃和150 ℃下加热1 h,番茄浆中顺式异构体的含量分别由未处理新鲜番茄中的6.1%增加至10.0%和56.2%[14]。
2 番茄红素的消化、吸收、代谢 2.1 番茄红素的消化、吸收番茄红素为脂溶性植物化合物,其消化、吸收与脂肪类似,其在血清中出现的时间也与其他膳食脂类相近[15]。已有研究发现,番茄红素的消化、吸收主要涉及口腔、胃和小肠3个部位[16]。当人体通过饮食摄入番茄红素后,番茄红素首先通过咀嚼从食物基质中释放(释放量 < 10%[17]),进入胃后转移到胃中乳液的脂相中[18]。随后在小肠内会与含有磷脂、游离脂肪酸、单酰甘油和胆汁盐的胶束结合在一起作为一个极性载体[19],通过被动扩散到达小肠黏膜细胞的刷状缘膜,在转运蛋白的协助下被吸收[15, 20-21]。
番茄红素的疏水性决定其难以溶解在水相中被吸收,新鲜番茄中的番茄红素穿过肠道屏障到达靶向细胞(即生物利用度)较低(0.1%–3.0%),小鼠小肠各段对新鲜番茄中番茄红素的吸收能力由强到弱为空肠、十二指肠、回肠[14, 22-23]。未吸收部分随食物基质继续进入大肠,而大肠上皮细胞对番茄红素无吸收,约70%的番茄红素在结肠发酵过程中释放[24]。体外发酵试验发现,番茄红素能够增加Roseburia、Subdoligranulum、Holdemanella的丰度,抑制Collinsella的丰度,经过24 h发酵后增加了乙酸、丙酸、乳酸的含量[25],这表明番茄红素可以被肠道菌群利用。
番茄红素的吸收程度机体年龄、性别、荷尔蒙状态、吸烟、酒精和其他食物成分的影响[8]。例如,老年人对于番茄红素的生物利用率较差[26];与富含多不饱和脂肪酸的玉米油相比,使用富含单不饱和脂肪的橄榄油烹饪番茄可以增加人体对番茄红素的吸收[20]。
2.2 番茄红素的代谢番茄红素在体内的分布与组织低密度脂蛋白受体数量、脂蛋白摄取量等因素有关[27]。番茄红素进入血液后输送至身体各部位,优先聚集在睾丸、肾上腺、肝脏和前列腺中[20]。番茄红素在不同组织中分布水平的差异很可能与脂蛋白受体和胆固醇转运体组织表达的差异有关[28]。
此外,番茄红素的代谢是通过酶促反应或者氧化裂解完成的。哺乳动物中主要的番茄红素切割酶是β-胡萝卜素-15, 15′-加氧酶(β-carotene-15, 15′-oxygenase, BCO1)和β-胡萝卜素-9′, 10′-双加氧酶(β-carotene-9′, 10′-dioxygenase, BCO2)[29]。但有研究认为番茄红素不能被BCO1切割裂解,而BCO2才是主要用于裂解番茄红素的酶,尤其是针对顺式异构体[8]。番茄红素在体内的氧化裂解主要源于吸烟、喝酒所产生的氧化应激。目前研究发现番茄红素的代谢产物有apo-lycopenals、apo-car-otenedials、apo-lycopenones、羧酸和环氧化合物等,它们大多具有生物活性。例如BCO2可以使番茄红素发生不对称裂解生成apo-10′-lycopenals (在人体血清中可检测到)、apo-10-lycopenol以及α-亚麻酸[30],α-亚麻酸却能够通过上调SIRT1 (脂质代谢的关键调节因子)表达以预防脂肪肝[31];番茄红素的中心裂解产物无环视黄酸可增强细胞间隙连接通讯以预防癌症[32]。虽然目前对番茄红素代谢物的研究已经有了突破性进展,但由于代谢存在个体差异性,代谢机制尚未明确。
3 番茄红素调控肠道菌群影响健康 3.1 番茄红素调控肠道菌群影响炎症发展摄入高脂肪饮食会引起肠道菌群紊乱,增加肠道通透性,导致炎症发生,因此肠道菌群与炎症有着密不可分的联系[33-34]。研究发现,肠道菌群能够影响机体炎症细胞的分化和细胞因子的产生,在炎症调节中发挥着重要作用[35]。而番茄红素能够改变肠道菌群组成,进而影响炎症发展。研究表明,补充番茄红素能使肠炎小鼠肠道菌群中双歧杆菌(Bifidobacterium)的比例由0恢复至1.0%[33]。有研究给予高脂肪饮食小鼠富含番茄红素的番茄粉,检测到高脂肪饮食小鼠粪便菌群中粘液螺旋体属(Mucispirillum)的丰度显著下降(P < 0.05),与其丰度呈正相关的小鼠血清瘦素水平下降,能减少肠黏膜中病原菌的扩张[29, 36-37];同时乳杆菌属(Lactobacillus)等菌属丰度的增加也被证明能减少黏膜损伤,阻止肠炎的发展[38]。
脂多糖(lipopolysaccharide, LPS)是一种强效内毒素,主要分布于革兰氏阴性菌的细胞壁中,能激活机体Toll样受体4 (TLR-4)诱导炎症反应[39]。而当已患结肠炎小鼠摄入番茄红素后,小鼠肠道菌群的α多样性得到了恢复。在菌属水平上,拟杆菌属(Bacteroides)、Escherichia、苏特氏菌(Sutterella)等革兰氏阴性菌的丰度显著下降(P < 0.05),LPS生成量减少,小鼠的血清LPS水平下降,结肠炎症状得到缓解[33]。
在日常饮食中补充番茄红素也能够有效预防炎症的发生。在常规饮食基础上给仔猪额外饲喂50 mg/kg番茄红素,通过Spearman相关性分析发现仔猪结肠内Selenomonas、Prevotellaceae_unclassified和Treponema_2、Megasphaera菌属的丰度下降与NRF2和IL-22、CLDN1的mRNA表达水平上升具有相关性,其中NRF2、CLDN1作为细胞抗氧化反应的调控因子能促进肠上皮细胞增殖,使其肠道绒毛排列更整齐,表面更光滑,结构更清晰,也降低仔猪患炎症风险[40-42]。
综上所述,番茄红素干预肠道炎症可能是通过降低肠道菌群中Bacteroides等革兰氏阴性菌的丰度、促进Lactobacillus等有益菌属的生长,减少LPS等致炎症因素,有效预防和抑制炎症的发生和发展。
3.2 番茄红素调控肠道菌群影响心血管疾病发展心血管疾病的主要病因是高脂血症,表现为血液总胆固醇(total cholesterol, TC)、甘油三酯(triglycerides, TG)、低密度脂蛋白胆固醇(low-density lipoprotein cholesterol, LDL-C)过高和高密度脂蛋白胆固醇(high-density lipoprotein cholesterol, HDL-C)浓度过低[43]。
肠道菌群在心血管疾病如动脉粥样硬化的发展中起着关键作用。研究发现,一些肠道菌群代谢产生的高浓度三甲基胺-N-氧化物(trimethylamine N-oxide, TMAO)会导致动脉粥样硬化的发生[35]。而番茄红素能够通过影响肠道菌群丰度减少这种有害物质的产生,从而阻止动脉粥样硬化的发展。给高脂肪饮食小鼠持续12周给予番茄红素,发现小鼠肠道菌群中Akkermansia的丰度明显增加,帮助恢复小鼠肠道黏膜完整性,改善葡萄糖-胰岛素稳态[44];而同时也发现小鼠血清TMAO水平以及TC、TG、LDL-C、HDL-C水平均发生下降,高脂血症症状减轻[45]。
在临床试验中发现摄入番茄红素可调节肠道菌群,进而影响心血管疾病的发展。中度肥胖成人每日摄入30 mg番茄红素,4周后可以使血液中低密度脂蛋白(LDL)含量平均减少130 mg/L,TG含量平均减少30 mg/L,同时发现试验对象肠道排泄物中放线菌门(Actinobacteria)的相对丰度由4.5%提升至7.12%,其中Bifidobacterium adolescentis、Bifidobacterium longum的相对丰度明显增加,但此研究中番茄红素、肠道菌群和血清TG、LDL之间的相关性未进行说明[46]。
综上所述,番茄红素能够促进肠道中Akkermansia等菌属的丰度增加,使高脂血症相关指标水平降低,心血管疾病发展的发展因此受到遏制。但摄入番茄红素对宿主肠道菌群改变以及对宿主心血管疾病发展的影响机制仍需深入探究。
3.3 番茄红素调控肠道菌群影响非酒精性脂肪肝发展非酒精性脂肪性肝病(nonalcoholic fatty liver disease, NAFLD)是一种与肥胖相关的疾病,临床表现包括肝细胞的TG积累和胰岛素抵抗,主要特征是血液中胰岛素、葡萄糖、极低密度脂蛋白(VLDL)和LDL的水平偏高。随着病情的加重,NAFLD可能演变为非酒精性脂肪性肝炎、肝硬化,最终导致肝癌[47],然而目前没有药物能够有效阻止这一发展,因此有必要进行膳食干预[29]。肝脏作为番茄红素的主要累积部位,研究表明其相关疾病的发展受到番茄红素摄入的影响。此前已有研究显示番茄红素被BCO2转化生成的α-亚麻酸可以预防NAFLD[31]。
番茄红素可以通过调节特定菌属丰度对NAFLD的发生和发展产生影响。在临床上,研究发现番茄红素摄入量与人体粪便菌群中Bifidobacterium丰度、肝脏脂肪生成量之间均具有剂量依赖性[46]。在动物试验中,Pearson相关性分析发现小鼠盲肠中肠杆菌科(Enterobacteriaceae)丰度的提高与血清LPS的增加相关,这促进小鼠肝脏TLR-4的表达,进而造成小鼠患肝脏疾病。而摄入番茄红素能通过调节肠道菌群改善高脂肪饮食小鼠肝脏抗氧化状态,从而阻止NAFLD的发展。主要表现为肠道菌群多样性增加,肠杆菌科(Enterobacteriaceae)丰度下降和Lactobacillus丰度提升,以及乙酸、丁酸生成量增加和两者生成量比值降低[34, 47-48]。人群流行病学调查也显示,摄入富含番茄红素的果蔬能使肠道菌群显示出较高的α多样性,患NAFLD等肝脏疾病的风险更低[49]。
综上所述,番茄红素能够提升Bifidobacterium等菌属的丰度,降低肠杆菌科(Enterobacteriaceae)所属分类下的菌属丰度,从而促进短链脂肪酸(short-chain fatty acids, SCFA)的生成和比例的改变,干预NAFLD的发展。动物试验、临床试验以及流行病学调查都为番茄红素能通过调控肠道菌群影响NAFLD发展的结论提供了支撑。
3.4 番茄红素调控肠道菌群影响癌症发展肠道菌群的改变与癌症的发展存在关联性。如Fusobacteria等菌属的丰度持续上升和Bifidobacterium、Lactobacillus等菌属的丰度持续下降会导致有毒细菌代谢产物增加、有益细菌代谢产物减少以及肠道组织屏障破坏,使直肠黏膜炎症逐步发展至直肠癌[50]。此外,在临床治疗中,也有通过补充Bifidobacterium重新激活肿瘤抑制基因的案例[51]。
番茄红素或能通过调节肠道菌群组成实现对癌症发展的干预。Bifidobacterium被证明具有肿瘤靶向性,即能依赖IFN-Ⅰ信号和T细胞并受IFN基因刺激因子调控而治疗肿瘤,能使小鼠肝脏肿瘤体积减少95%[30];Lactobacillus brevis MK05的代谢物也显示出抗肿瘤作用[52]。另有一项研究表明,Bifidobacterium longum和番茄红素的共同使用能够使小鼠结肠癌发病率由80%降至13%,番茄红素能通过阻止炎症发生和对Bifidobacterium longum的调节影响癌症的发展[53]。
综上所述,番茄红素可以通过提高Bifidobacterium、Lactobacillus等菌属,增加有益代谢产物,阻止炎症向癌症进一步发展,但番茄红素改变肠道菌群对影响癌症发展的作用机制仍需进一步研究。
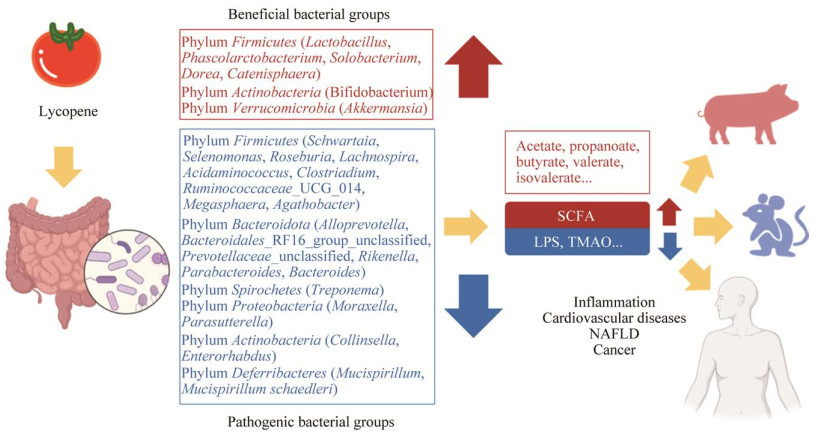
4 总结与展望通过综述相关研究成果,表明膳食摄入番茄红素主要能够提高宿主体内厚壁菌门(Firmicutes)、放线菌门(Actinobacteria)、疣微菌门(Verrucomicrobia)等菌门中部分菌属丰度,降低厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、螺旋体门(Spirochetes)、变形菌门(Proteobacteria)、放线菌门(Actinobacteria)和脱铁杆菌门(Deferribacteres)等菌属的丰度,并提升菌群多样性,通过增加各种SCFA和减少LPS、TMAO等肠道菌群代谢产物,抑制炎症、心血管疾病、非酒精性脂肪肝、癌症的治病基因表达,从而对病情发展产生影响,总结归纳为图 1。因此番茄红素作为番茄中典型的生物活性物质,对于功能食品、药物生产开发有巨大价值。但仍有一些研究值得深入探索。(1) 番茄红素在体内、体外都会发生不同程度的顺式异构化,有必要进一步研究顺式、反式异构体各自对疾病干预的作用,以指导未来番茄制品的加工技术与工艺。(2) 番茄红素已被证实对其他疾病如老年黄斑变性、男性不育症等有干预作用,肠道菌群是否也参与其中?值得进一步探讨和明悉,从而发挥番茄红素对人类健康影响的优势。(3) 以上文献表明番茄红素能够调控肠道菌群对宿主健康产生影响,但番茄红素对肠道菌群的调控机理以及因其改变的肠道菌群各菌属对各类疾病发展的干预机制仍需深入探索。(4) 有待改良现有产品使番茄红素最大程度优化肠道菌群组成,以充分发挥其对健康的有利影响。这对于番茄功能性食品的开发具有重要指导作用。
|
| 图 1 肠道菌群介导的番茄红素对宿主疾病的干预 Figure 1 Intestinal microbiota-mediated lycopene intervention on host diseases. |
除了番茄红素,番茄中还有许多营养成分也具有预防和改善疾病作用。如番茄中的鼠李糖半乳糖酸果胶,能够通过促进消炎共生菌群发挥免疫调节作用[34]。而番茄皮中最丰富的酚类物质羟基肉桂酸、羟基苯甲酸及其衍生物和黄酮类化合物无法被消化,可为肠道菌群所利用,被发酵成为更简单的、具有更强生物活性的酚类[35]。此外,番茄植株的叶片中富含的叶绿素也能够增加肠道菌群多样性[54]。因此,肠道菌群介导的这些功能性成分对健康的影响作用也是未来值得深入探究的方向。
| [1] | COLLINS EJ, BOWYER C, TSOUZA A, CHOPRA M. Tomatoes: an extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation[J]. Biology, 2022, 11(2): 239 DOI:10.3390/biology11020239. |
| [2] | LEE J, LIM JW, KIM H. Lycopene inhibits IL-6 expression by upregulating NQO1 and HO-1 via activation of Nrf2 in ethanol/lipopolysaccharide-stimulated pancreatic acinar cells[J]. Antioxidants (Basel, Switzerland), 2022, 11(3): 519. |
| [3] | SOCHOCKA M, DONSKOW-ŁYSONIEWSKA K, DINIZ BS, KURPAS D, BRZOZOWSKA E, LESZEK J. The gut microbiome alterations and inflammation-driven pathogenesis of alzheimer's disease—a critical review[J]. Molecular Neurobiology, 2019, 56(3): 1841-1851 DOI:10.1007/s12035-018-1188-4. |
| [4] | de VOS WM, TILG H, van HUL M, CANI PD. Gut microbiome and health: mechanistic insights[J]. Gut, 2022, 71(5): 1020-1032 DOI:10.1136/gutjnl-2021-326789. |
| [5] | SHREINER AB, KAO JY, YOUNG VB. The gut microbiome in health and in disease[J]. Current Opinion in Gastroenterology, 2015, 31(1): 69-75 DOI:10.1097/MOG.0000000000000139. |
| [6] | RAJPUT SA, LIANG SJ, WANG XQ, YAN HC. Lycopene protects intestinal epithelium from deoxynivalenol-induced oxidative damage via regulating Keap1/Nrf2 signaling[J]. Antioxidants (Basel, Switzerland), 2021, 10(9): 1493. |
| [7] | LEH HE, LEE LK. Lycopene: a potent antioxidant for the amelioration of type II diabetes mellitus[J]. Molecules (Basel, Switzerland), 2022, 27(7): 2335 DOI:10.3390/molecules27072335. |
| [8] | CASEIRO M, ASCENSO A, COSTA A, CREAGH-FLYNN J, JOHNSON M, SIMÕES S. Lycopene in human health[J]. LWT, 2020, 127: 109323 DOI:10.1016/j.lwt.2020.109323. |
| [9] |
李京, 惠伯棣. 番茄红素在体内代谢中的几何异构体组成变化[J]. 食品科学, 2008, 29(9): 591-594.
DOI:10.3321/j.issn:1002-6630.2008.09.142 LI J, HUI BD. Variation in geometrical isomer composition of lycopene in rat body[J]. Food Science, 2008, 29(9): 591-594 (in Chinese). |
| [10] | MORAN NE, THOMAS-AHNER JM, SMITH JW, SILVA C, HASON NA, ERDMAN JW, CLINTON SK. β-carotene oxygenase 2 genotype modulates the impact of dietary lycopene on gene expression during early TRAMP prostate carcinogenesis[J]. The Journal of Nutrition, 2022, 152(4): 950-960 DOI:10.1093/jn/nxab445. |
| [11] | VITUCCI D, AMORESANO A, NUNZIATO M, MUOIO S, ALFIERI A, ORIANI G, SCALFI L, FRUSCIANTE L, RIGANO MM, PUCCI P, FONTANA L, BUONO P, SALVATORE F. Nutritional controlled preparation and administration of different tomato purées indicate increase of β-carotene and lycopene isoforms, and of antioxidant potential in human blood bioavailability: a pilot study[J]. Nutrients, 2021, 13(4): 1336 DOI:10.3390/nu13041336. |
| [12] | LI N, WU XT, ZHUANG W, XIA L, CHEN Y, WU CC, RAO ZY, DU L, ZHAO R, YI MS, WAN QY, ZHOU Y. Tomato and lycopene and multiple health outcomes: umbrella review[J]. Food Chemistry, 2021, 343: 128396 DOI:10.1016/j.foodchem.2020.128396. |
| [13] | CIRIMINNA R, FIDALGO A, MENEGUZZO F, ILHARCO LM, PAGLIARO M. Lycopene: emerging production methods and applications of a valued carotenoid[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 643-650. |
| [14] |
于颖, 张维, 谢凡, 顾欣哲, 吴金鸿, 王正武. 改善番茄红素生物利用度的研究进展[J]. 食品科学, 2019, 40(19): 346-352.
DOI:10.7506/spkx1002-6630-20181011-094 YU Y, ZHANG W, XIE F, GU XZ, WU JH, WANG ZW. Progress in the improvement of lycopene bioavailability[J]. Food Science, 2019, 40(19): 346-352 (in Chinese). |
| [15] | CLARK RM, YAO LL, SHE L, FURR HC. A comparison of lycopene and canthaxanthin absorption: using the rat to study the absorption of non-provitamin a carotenoids[J]. Lipids, 1998, 33(2): 159-163 DOI:10.1007/s11745-998-0191-0. |
| [16] | LIANG XP, MA CC, YAN XJ, LIU XB, LIU FG. Advances in research on bioactivity, metabolism, stability and delivery systems of lycopene[J]. Trends in Food Science & Technology, 2019, 93: 185-196. |
| [17] | GOUPY P, GENOT C, HAMMAZ F, HALIMI C, CARIS-VEYRAT C, BOREL P. Mechanisms governing the transfer of pure and plant matrix carotenoids toward emulsified triglycerides[J]. Molecular Nutrition & Food Research, 2020, 64(7): e1900911. |
| [18] | DEGROU A, GEORGÉ S, RENARD CMGC, PAGE D. Physicochemical parameters that influence carotenoids bioaccessibility from a tomato juice[J]. Food Chemistry, 2013, 136(2): 435-441 DOI:10.1016/j.foodchem.2012.08.065. |
| [19] | NAGAO A. Absorption and metabolism of dietary carotenoids[J]. BioFactors (Oxford, England), 2011, 37(2): 83-87 DOI:10.1002/biof.151. |
| [20] | CLARK RM, YAO LL, SHE L, FURR HC. A comparison of lycopene and astaxanthin absorption from corn oil and olive oil emulsions[J]. Lipids, 2000, 35(7): 803-806 DOI:10.1007/s11745-000-0589-8. |
| [21] | TOTI E, CHEN CO, PALMERY M, VILLAÑO VALENCIA D, PELUSO I. Non-provitamin A and provitamin A carotenoids as immunomodulators: recommended dietary allowance, therapeutic index, or personalized nutrition?[J]. Oxidative Medicine and Cellular Longevity, 2018, 2018: 4637861. |
| [22] | DURING A, HARRISON EH. An in vitro model to study the intestinal absorption of carotenoids[J]. Food Research International, 2005, 38(8/9): 1001-1008. |
| [23] |
朱金芳, 马雪红, 陈旭. 番茄红素脂质体体外释放及在体肠吸收特性研究[J]. 新疆医科大学学报, 2018, 41(10): 1283-1286.
DOI:10.3969/j.issn.1009-5551.2018.10.021 ZHU JF, MA XH, CHEN X. Studies on release in vitro and intestinal absorption in situ of lycopene liposomes[J]. Journal of Xinjiang Medical University, 2018, 41(10): 1283-1286 (in Chinese). |
| [24] | GOÑI I, SERRANO J, SAURA-CALIXTO F. Bioaccessibility of beta-carotene, lutein, and lycopene from fruits and vegetables[J]. Journal of Agricultural and Food Chemistry, 2006, 54(15): 5382-5387 DOI:10.1021/jf0609835. |
| [25] | DAI ZQ, LI ZX, SHI EJ, NIE MM, FENG L, CHEN GJ, GAO RC, ZENG XX, LI DJ. Study on the interaction between four typical carotenoids and human gut microflora using an in vitro fermentation model[J]. Journal of Agricultural and Food Chemistry, 2022, 70(42): 13592-13601 DOI:10.1021/acs.jafc.2c03464. |
| [26] | KONG KW, KHOO HE, PRASAD KN, ISMAIL A, TAN CP, RAJAB NF. Revealing the power of the natural red pigment lycopene[J]. Molecules (Basel, Switzerland), 2010, 15(2): 959-987 DOI:10.3390/molecules15020959. |
| [27] | DURAIRAJANAYAGAM D, AGARWAL A, ONG C, PRASHAST P. Lycopene and male infertility[J]. Asian Journal of Andrology, 2014, 16(3): 420-425 DOI:10.4103/1008-682X.126384. |
| [28] | PETYAEV IM. Lycopene deficiency in aging and cardiovascular disease[J]. Oxidative Medicine and Cellular Longevity, 2016, 2016: 1-6. |
| [29] | XIA H, LIU C, LI CC, FU MB, TAKAHASHI S, HU KQ, AIZAWA K, HIROYUKI S, WU GJ, ZHAO LP, WANG XD. Dietary tomato powder inhibits high-fat diet-promoted hepatocellular carcinoma with alteration of gut microbiota in mice lacking carotenoid cleavage enzymes[J]. Cancer Prevention Research (Philadelphia, Pa), 2018, 11(12): 797-810 DOI:10.1158/1940-6207.CAPR-18-0188. |
| [30] | SONG XY, LUO YH, MA LJ, HU XS, SIMAL-GANDARA J, WANG LS, BAJPAI VK, XIAO JB, CHEN F. Recent trends and advances in the epidemiology, synergism, and delivery system of lycopene as an anti-cancer agent[J]. Seminars in Cancer Biology, 2021, 73: 331-346 DOI:10.1016/j.semcancer.2021.03.028. |
| [31] | CHUNG J, KOO K, LIAN FZ, HU KQ, ERNST H, WANG XD. Apo-10′-lycopenoic acid, a lycopene metabolite, increases sirtuin 1 mRNA and protein levels and decreases hepatic fat accumulation in ob/ob mice[J]. The Journal of Nutrition, 2012, 142(3): 405-410 DOI:10.3945/jn.111.150052. |
| [32] | MEIN JR, LIAN FZ, WANG XD. Biological activity of lycopene metabolites: implications for cancer prevention[J]. Nutrition Reviews, 2008, 66(12): 667-683 DOI:10.1111/j.1753-4887.2008.00120.x. |
| [33] | ZHAOBT, WU JB, LI JH, BAI Y, LUO Y, JI B, XIA B, LIU ZG, TAN XT, LV JY, LIU XB. Lycopene alleviates DSS-induced colitis and behavioral disorders via mediating microbes-gut-brain axis balance[J]. Journal of Agricultural and Food Chemistry, 2020, 68(13): 3963-3975 DOI:10.1021/acs.jafc.0c00196. |
| [34] | LI CC, LIU C, FU MB, HU KQ, AIZAWA K, TAKAHASHI S, HIROYUKI S, CHENG JR, von LINTIG J, WANG XD. Tomato powder inhibits hepatic steatosis and inflammation potentially through restoring SIRT1 activity and adiponectin function independent of carotenoid cleavage enzymes in mice[J]. Molecular Nutrition & Food Research, 2018, 62(8): e1700738. |
| [35] | BRANDSMA E, KLOOSTERHUIS NJ, KOSTER M, DEKKER DC, GIJBELS MJJ, van der VELDEN S, RÍOS-MORALES M, van FAASSEN MJR, LORETI MG, de BRUIN A, FU JY, KUIPERS F, BAKKER BM, WESTERTERP M, de WINTHER MPJ, HOFKER MH, van de SLUIS B, KOONEN DPY. A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis[J]. Circulation Research, 2019, 124(1): 94-100 DOI:10.1161/CIRCRESAHA.118.313234. |
| [36] | LOY A, PFANN C, STEINBERGER M, HANSON B, HERP S, BRUGIROUX S, GOMES NETO JC, BOEKSCHOTEN MV, SCHWAB C, URICH T, RAMER-TAIT AE, RATTEI T, STECHER B, BERRY D. Lifestyle and horizontal gene transfer-mediated evolution of Mucispirillum schaedleri, a core member of the murine gut microbiota[J]. mSystems, 2017, 2(1): e00171-e00116. |
| [37] | WANG J, WANG Z, LI B, QIANG Y, YUAN T, TAN XT, WANG ZH, LIU ZG, LIU XB. Lycopene attenuates western-diet-induced cognitive deficits via improving glycolipid metabolism dysfunction and inflammatory responses in gut-liver-brain axis[J]. International Journal of Obesity, 2019, 43(9): 1735-1746 DOI:10.1038/s41366-018-0277-9. |
| [38] | CAESAR R, TREMAROLI V, KOVATCHEVADATCHARY P, CANI PD, BÄCKHED F. Crosstalk between gut microbiota and dietary lipids aggravates WAT inflammation through TLR signaling[J]. Cell Metabolism, 2015, 22(4): 658-668 DOI:10.1016/j.cmet.2015.07.026. |
| [39] | FULOP T, WITKOWSKI JM, BOURGADE K, KHALIL A, ZERIF E, LARBI A, HIROKAWA K, PAWELEC G, BOCTI C, LACOMBE G, DUPUIS G, FROST EH. Can an infection hypothesis explain the beta amyloid hypothesis of alzheimer's disease?[J]. Frontiers in Aging Neuroscience, 2018, 10: 224 DOI:10.3389/fnagi.2018.00224. |
| [40] | MENGQW, ZHANG YM, LI JB, SHI BM, MA QQ, SHAN AS. Lycopene affects intestinal barrier function and the gut microbiota in weaned piglets via antioxidant signaling regulation[J]. The Journal of Nutrition, 2022, 152(11): 2396-2408 DOI:10.1093/jn/nxac208. |
| [41] | SOVRAN B, LOONEN LMP, LU P, HUGENHOLTZ F, BELZER C, STOLTE EH, BOEKSCHOTEN MV, van BAARLEN P, KLEEREBEZEM M, de VOS P, DEKKER J, RENES IB, WELLS JM. IL-22-STAT3 pathway plays a key role in the maintenance of ileal homeostasis in mice lacking secreted mucus barrier[J]. Inflammatory Bowel Diseases, 2015, 21(3): 531-542 DOI:10.1097/MIB.0000000000000319. |
| [42] | DIAS MC, VIEIRALVES NFL, GOMES MIFV, SALVADORI DMF, RODRIGUES MAM, BARBISAN LF. Effects of lycopene, synbiotic and their association on early biomarkers of rat colon carcinogenesis[J]. Food and Chemical Toxicology, 2010, 48(3): 772-780 DOI:10.1016/j.fct.2009.12.003. |
| [43] | HE WS, JIA CS, YANG YB, MA Y, ZHANG XM, FENG B, JIN J. Cholesterol-lowering effects of plant steryl and stanyl laurate by oral administration in mice[J]. Journal of Agricultural and Food Chemistry, 2011, 59(9): 5093-5099 DOI:10.1021/jf104031e. |
| [44] | BALDWIN J, COLLINS B, WOLF PG, MARTINEZ K, SHEN W, CHUANG CC, ZHONG W, COONEY P, COCKRELL C, CHANG E, GASKINS HR, McINTOSH MK. Table grape consumption reduces adiposity and markers of hepatic lipogenesis and alters gut microbiota in butter fat-fed mice[J]. The Journal of Nutritional Biochemistry, 2016, 27: 123-135 DOI:10.1016/j.jnutbio.2015.08.027. |
| [45] | WU T, GAO YF, HAO JY, YIN JJ, LI W, GENG JT, LIU R, SUI WJ, ZHANG M. Lycopene, amaranth, and sorghum red pigments counteract obesity and modulate the gut microbiota in high-fat diet fed C57BL/6 mice[J]. Journal of Functional Foods, 2019, 60: 103437 DOI:10.1016/j.jff.2019.103437. |
| [46] | WIESE M, BASHMAKOV Y, CHALYK N, NIELSEN DS, KRYCH Ł, KOT W, KLOCHKOV V, PRISTENSKY D, BANDALETOVA T, CHERNYSHOVA M, KYLE N, PETYAEV I. Prebiotic effect of lycopene and dark chocolate on gut microbiome with systemic changes in liver metabolism, skeletal muscles and skin in moderately obese persons[J]. BioMed Research International, 2019, 2019: 4625279. |
| [47] | GARCÍA-ALONSO FJ, GONZÁLEZ-BARRIO R, MARTÍN-POZUELO G, HIDALGO N, NAVARRO-GONZÁLEZ I, MASUERO D, SOINI E, VRHOVSEK U, PERIAGO MJ. A study of the prebiotic-like effects of tomato juice consumption in rats with diet-induced non-alcoholic fatty liver disease (NAFLD)[J]. Food & Function, 2017, 8(10): 3542-3552. |
| [48] | SINGH DP, KHARE P, ZHU J, KONDEPUDI KK, SINGH J, BABOOTA RK, BOPARAI RK, KHARDORI R, CHOPRA K, BISHNOI M. A novel cobiotic-based preventive approach against high-fat diet-induced adiposity, nonalcoholic fatty liver and gut derangement in mice[J]. International Journal of Obesity, 2016, 40(3): 487-496 DOI:10.1038/ijo.2015.197. |
| [49] | FRANKENFELD CL, HULLAR MAJ, MASKARINEC G, MONROE KR, SHEPHERD JA, FRANKE AA, RANDOLPH TW, WILKENS LR, BOUSHEY CJ, Le MARCHAND L, LIM U, LAMPE JW. The gut microbiome is associated with circulating dietary biomarkers of fruit and vegetable intake in a multiethnic cohort[J]. Journal of the Academy of Nutrition and Dietetics, 2022, 122(1): 78-98 DOI:10.1016/j.jand.2021.05.023. |
| [50] | SUN J, KATO I. Gut microbiota, inflammation and colorectal cancer[J]. Genes & Diseases, 2016, 3(2): 130-143. |
| [51] | HELMINK BA, KHAN MAW, HERMANN A, GOPALAKRISHNAN V, WARGO JA. The microbiome, cancer, and cancer therapy[J]. Nature Medicine, 2019, 25(3): 377-388. |
| [52] | SINGLA RK, WANG XY, GUNDAMARAJU R, JOON S, TSAGKARIS C, BEHZAD S, KHAN J, GAUTAM R, GOYAL R, RAKMAI J, DUBEY AK, SIMAL-GANDARA J, SHEN BR. Natural products derived from medicinal plants and microbes might act as a game-changer in breast cancer: a comprehensive review of preclinical and clinical studies[J]. Critical Reviews in Food Science and Nutrition, 2022: 1-45. |
| [53] | VALADEZ-BUSTOS N, ESCAMILLA-SILVA EM, GARCÍA-VÁZQUEZ FJ, GALLEGOS-CORONA MA, AMAYA-LLANO SL, RAMOS-GÓMEZ M. Oral administration of microencapsulated B. longum BAA-999 and lycopene modulates IGF-1/IGF-1R/IGFBP3 protein expressions in a colorectal murine model[J]. International Journal of Molecular Sciences, 2019, 20(17): 4275. |
| [54] | LI YY, CUI Y, LU F, WANG X, LIAO XJ, HU XS, ZHANG Y. Beneficial effects of a chlorophyll-rich spinach extract supplementation on prevention of obesity and modulation of gut microbiota in high-fat diet-fed mice[J]. Journal of Functional Foods, 2019, 60: 103436. |
 2023, Vol. 63
2023, Vol. 63